Video Views and Reviews: Neurulation and the Fashioning of the Vertebrate Central Nervous System
The central nervous system (CNS) is the first adult organ system to appear during vertebrate development, and the process of its emergence is commonly called neurulation. Such biological “urgency” is perhaps not surprising given the structural and functional complexity of the CNS and the importance of neural function to adaptive behavior and individual survival.
Soon after an egg is fertilized, it is subdivided into many cells, and those cells are rearranged in a process called gastrulation, which begins the formation of the CNS. In fact, neurulation seems to begin before gastrulation is completed, and the two processes appear seamlessly joined, suggesting that many of the cells and underlying mechanisms are involved in both processes. Thus, students wishing to understand neurulation should begin their study with a review of gastrulation and its mechanisms using one of several text books (e.g., Gilbert, 2003) or an excellent monograph (Stern, 2004). They might also find useful a recent review of videos and published papers dealing with the subject (Watters, 2005).
NEURULATION IN XENOPUS
Amphibian embryos provide possibly the best reference material with which to begin a study of neurulation, if only because their early development has been extensively examined. In the Xenopus frog, the early external events are documented by an excellent time-lapse video (Keller, 2002; see also, Keller and Shook, 2004, http://www.gastrulation.org/Movie13_1.mov). The film depicts neurulation from a perspective above the dorsal surface of the embryo (Figure 1) and nicely illustrates both the continuity of neurulation and gastrulation (ending as the video begins) and the correlation of each process with anterior–posterior elongation of the embryo. A detailed tutorial on amphibian development, including additional videos from the Keller lab, is available at Jeff Hardin's Web site (http://worms.zoology.wisc.edu/frogs/welcome.html).1 1Unfortunately, the vertebrate embryos most available for study are also relatively yolky and opaque. Correspondingly, internal features of gastrulation and neurulation are difficult to follow in living material. In this regard, another film of Xenopus development at the Hardin Web site (http://worms.zoology.wisc.edu/frogs/gastxen/gastxen_sagview.html) is noteworthy for its interior view of gastrulation along the sagittal plane (midline) of a living embryo.
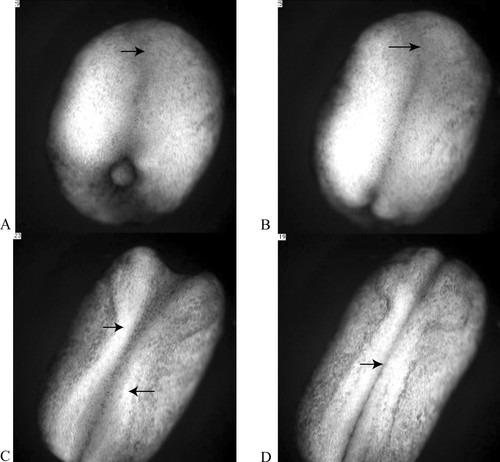
Figure 1. Four “stages” of amphibian neurulation, as viewed from above the dorsal surface of a Xenopus embryo. The future head of the embryo is oriented toward the top of each frame, its tail at the bottom. As development proceeds, the embryo elongates along its anterior–posterior axis, and a flattened region of the dorsal epithelium, or neural plate, appears on its dorsal surface (A, arrow). As neurulation proceeds, a neural groove becomes evident along the midline of the plate, with a widened depression (B, arrow) at the anterior end (B). The lateral edges of the plate (C, arrows) become prominent and fold in toward the midline of the embryo. At the end of neurulation, the neural folds become contiguous along most of the dorsal midline and begin to fuse (C, arrow). The sequence in A–D lasts about five seconds and represents just over four hours of development. The entire video, which represents approximately 15 hours of development, may be viewed online at http://www.sciencemag.org/content/vol298/issue5600/images/data/1950/DC1/1079478s1.mov.
In brief, during neurulation the CNS begins as a somewhat flattened layer of epithelial cells on the upper or dorsal surface of the amphibian embryo. This flattened layer is called the neural plate (Figure 1A) and appears with the completion of gastrulation. With the onset of neurulation, the embryo also begins to elongate along its anterior–posterior axis and become restricted in girth. As neurulation progresses, elongation continues, and the lateral edges of the plate, or neural folds, become prominent and begin to bend toward each other to form a tube (Figure 1B). As the neural folds of the plate close, the dorsal midline of the embryo begins to sink somewhat below the surface, and in making contact, the central depression in the plate becomes more distinctive (Figure 1C). As they become contiguous, the folds fuse (Figure 1D), producing a hollow neural tube and a continuous dorsal epithelium, which effectively displaces the neural tube into the interior of the embryo. After separation from the overlying epithelium, the neural tube thickens differentially as various regions begin to form different subregions of the brain and spinal cord.
To understand how neurulation comes about, students should be encouraged to review the Keller video at the Hardin Web site and to discuss the cellular biology of gastrulation and neurulation (cf. Keller and Davidson, 2004). Doing so will likely generate numerous questions. For example, to what extent might elongation of the embryonic axis result from the epibolic and involutional movements characteristic of amphibian gastrulation? Might these internal movements produce tension in the overlying surface epithelial layer that initiates lateral folding (cf. Keller, 2002)? How might changes in the shape, adhesion, and relative position of the epithelial cells, for example, contribute to the folding of the neural plate and, eventually, to the closure of the neural tube? Could the convergence of dorsal plate cells toward the midline and their intercalation also produce some of the axial extension observed in embryos at this stage of development, in a process called “convergent extension” (Keller and Davidson, 2004).2 2Convergent extension is one of the more important developmental mechanisms underlying morphogenesis, and its three-dimensional features are difficult to illustrate to introductory students with static diagrams. Fortunately, an excellent article (Glickman et al., 2003) and 18 videos (http://dev.biologists.org/cgi/content/full/130/5/873/DC1) illustrate this process in wild-type and mutant zebrafish development. Unfortunately for the purposes of this Feature, however, the structure being examined and illustrated is the notochord, the embryonic forerunner of the vertebral column, which is not part of the CNS and which is derived from mesodermal cells that entered the embryo during gastrulation.
In reviewing the Keller video and considering these questions, students may generate many questions of their own, more than can be currently answered. Fortunately, an earlier article by Davidson and Keller (1999) describes the medial movement of neural cells, their intercalation and convergent extension, and provides important clues and some answers to underlying mechanisms. Unfortunately, the videos accompanying this article will be difficult for most undergraduates to appreciate, because they depict the behavior of loosely associated cells, called neural crest cells, photographed at high magnification, under low light conditions, and without spatial context. Given these imaging limitations, it's very difficult to visualize how their behavior is related to neural fold formation and closure, although the transverse sections examined by confocal microscopy provide excellent static images for temporal reconstruction (Davidson and Keller, 1999). More advanced students might enjoy discussing the results and the combined application of in situ RNA hybridization, confocal fluorescence microscopy, and videomicroscopy to correlate gene expression, cell movement, and neural tube formation.
Students who question how convergent extension and related cell movements might be coordinated will be interested in an article (with videos) describing the effect of Dishevelled (Dsh) on neural tube closure in Xenopus embryos (Wallingford and Harland, 2002). Figure 2 depicts the comparative development of embryos that had been injected with a mutant Dsh alongside embryos from the same population of fertilized eggs that had not been injected. All of the embryos began neurulation at about the same time, and all exhibited neural folds that became prominent and began to converge. The neural tubes of half of the injected embryos and all of the uninjected controls closed, although the rate of closure seemed faster in control embryos than in injected ones (compare the right-hand and middle panels in Figure 2). Half of the injected embryos, however, exhibited neural folds that failed to close (left-hand panel of embryos in Figure 2). Interestingly, the embryos with neural tubes that failed to close also seemed shorter and more stubby than the controls, and some students might immediately postulate a mutant disruption of convergent extension. To test this hypothesis, students could examine the sectioned material and other videos described in the article and discuss the details of mutant Dsh dysfunction. Other students might wonder how a mutant protein could exert partial effects, preventing closure in some embryos but not in others, and discussion of these results could lead to a fruitful exploration of 1) how “mutant effects” are produced using injection of mutant material into organisms such as Xenopus that lack collections of mutant strains, using injection of mutant material; 2) how mutant effects would occur in a “dominant negative” manner; and 3) how a spectrum of phenotypes might result from translational competition of the wild-type and mutant message. (Although unstated by the authors, the injected material is likely mRNA [Sive et al., 2000].) Somewhat more critical students also might wonder whether saline-injected, rather than uninjected, embryos would have provided better, more reliable controls.
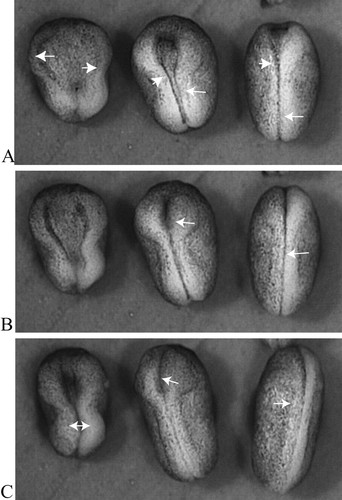
Figure 2. A dorsal view of three neurulating Xenopus embryos between the midneural stage 16 (A) and the neural tube stage 21 (C). The embryo on the left is expressing Xdd1, an injected mutant form of Dishevelled and fails to close its tube (open-NT). The middle embryo is also expressing Xdd1 but successfully closes its neural tube (closed-NT) while lagging behind the control. The embryo on the right is an uninjected control. The arrows indicate neural folds in various degrees of closure. Although only relative developmental times are provided (in the form of “stages”), the same population of embryos was subdivided, with one receiving an injection of mutant Xdd1 and the other behaving as an uninjected control; both subpopulations were reared under identical conditions. The time elapsed in A–C is approximately four hours. The entire video may be viewed at http://dev.biologists.org/content/vol129/issue24/images/data/5815/DC1/MOVIE1.mov.
Students who have taken a course in developmental biology should recognize Dishevelled as a downstream signaling peptide in the Wingless-int (Wnt) pathway. They would thus be aware of this pathway's important role in establishing tissue boundaries in Drosophila and Caenorhabditis elegans embryos, via activation of β-catenin, a putative transcription factor regulating cell division (cf. Gilbert, 2003). Because Dsh and β-catenin also help establish the dorsal axis of very young Xenopus embryos (Miller et al., 1999), and thereby affect the later development of such dorsal structures as the neural tube, some students might wonder how an injected, mutant form of Dsh could disrupt neurulation without apparently exerting a more global disruptive effect on axis formation. More thoughtful developmental biology students will recognize this paradox as another instance involving the timing of developmental events, where critical cues exert subtle effects well in advance of obvious phenotypic outcomes. Unfortunately, the authors do not indicate when mutant Dsh was injected into their Xenopus embryos, but useful discussion could be devoted to consideration of two hypothetical if/then scenarios. If injections occurred after the dorsal axis had been set, then the paradox is easily resolved and requires only a recognition that once axis formation is determined, subsequent developmental events along the axis are often inevitable and unchangeable. (Of course, such discussion quickly begs the more interesting question as to how exactly stable determination comes about and is maintained.) If, however, injection occurred right after fertilization, at the time the dorsal axis was being determined, then a different, but equally interesting, discussion would center on the developmental importance of maternal proteins and their localization in early Xenopus development as well as the timing of “new” mRNA synthesis. Cell biology students, however, might be more familiar with β-catenin's role as a linker peptide anchoring cytoskeletal elements to integral plasma membrane proteins called integrins and as a regulator of cytoskeletal protein phosphorylation (Lodish et al., 2004). A fruitful dialogue between these two groups of students might well produce some additional hypotheses how Dsh could regulate neurulation, both through differential growth and tissue delimitation as well as through those polarized changes in cell shape and attachment thought to be important for convergent extension.
NEURULATION IN FISH
Neurulation in bony fish seems to occur in much the same way as it does in amphibians, but the neural folds are much less prominent and form a nontubular thickened tissue often called a neural keel (which appears wedge-shaped in cross-section). The keel gradually rounds up into a solid neural rod and not a neural tube. The neural rod then forms a hollow core and tubelike structure (Langland and Kimmel, 1997). Much less is known about this “secondary” form of neurulation, although the Wnt pathway and β-catenin are implicated in its regulation (Gilbert, 2003). An interesting discussion for students in an intermediate-level course in developmental biology could be devoted to exploring mechanistic variations between the two forms of neurulation.
Any study of neurulation in bony fishes would likely involve genetically tractable zebrafish embryos, which are easily raised in the laboratory and increasingly used for developmental studies. Students could begin their study with a paper (and set of videos) describing the contribution of polarized cell division to keel formation, which also provides a succinct, well-illustrated summary of neurulation (Geldmacher-Voss et al., 2003). These authors used zebrafish strains stably expressing histone2a chimeras fused with green fluorescent protein (GFP) to follow chromosome movements. To monitor the orientation of mitotic spindles during neurulation, they injected mRNA encoding a tau-GFP chimera; tau is a microtubule (MT)-associated protein (MAP) responsible for stabilizing bundles or fibers of MTs.
As the neural keel forms, cell divisions initially occur within the neural epithelium with mitotic spindles oriented at right angles to the neural plate. As daughter cells accumulate in a vertical direction, the keel thickens. The plane of cell divisions then rotates 90° such that in subsequent divisions, daughter cells are produced parallel with the surface and oriented at right angles to the developing anterior–posterior axis of the embryo. These features are illustrated in Figure 3, A–C, which was taken from a video of sequences lasting seven seconds, documenting ∼102 minutes of development.
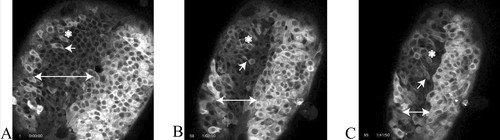
Figure 3. The convergence of neuroepithelial cells toward the dorsal midline of a zebrafish embryo during the later stages of neurulation. Shortening the length of the double-headed arrow indicates convergence of the same epithelial regions toward the midline. During the hour that elapses between A and B, the cell indicated by the single-headed arrow undergoes mitosis and produces two daughter cells oriented more or less at a right angle to the midline. The same daughter cell is marked by the single-headed arrow in B and C, and its displacement toward the midline may be gauged in reference to the asterisk, which marks the position of a neighboring cell in A. Fluorescence was generated by injecting the embryo at the two-cell stage of development with an mRNA coding for a chimera of GFP and tau, a MAP that stabilizes MT associations. The cells on the right side of the embryo expressed tau:GFP more strongly than those on the left side. The video may be viewed online at http://dev.biologists.org/content/vol130/issue16/images/data/3767/DC1/MOVIE1.MPG.
What is especially evident in the video (and Figure 3) is the extension of the neural plate along the anterior–posterior embryonic axis and concomitant shrinkage of the plate at right angles to this axis, which accompanies keel formation. This convergent extension of the keel is visually dramatic because greater amounts of tau:GFP chimeras are produced in cells along the left and, even more so, the right margins of the plate. The authors claim most cell divisions at this stage of neurulation are polarized (single arrows) and contribute to convergent extension of the keel. Students should be encouraged to assess this claim by mapping the orientations and mitotic fates of other apical cells and then discussing how such cells could extend the embryonic axis through their convergence at the midline. Some will likely agree that certain cell divisions seem to move daughter cells closer to the keel midline (single arrow, Figure 3, A and B), whereas others may argue that cytokinesis is producing daughter cells with other orientations in the apical layer and that these cells are being displaced toward the midline by different mechanisms. Curious students may wonder whether the brightly fluorescent cells on the right-hand side of the neural plate are somehow forming a “cleft” in the keel. To determine whether such a cleft is forming, they should be encouraged to examine other videos in the series and to speculate how such a cleft might be formed. More advanced students might also explore the article and other videos in greater detail, examining the localization of GFP chimeras with various cell junction markers. Further, they might consider what would be the effects of injecting antisense RNAs (“morpholinos”) for cell junction markers on both their localization and neural keel formation.
This article and others by Keller and his associates on Xenopus neurulation might also provide the basis for an extended journal club discussion of the cellular bases for neural morphogenesis.
As always, I welcome your comments about this Feature and your suggestions of other published, peer-reviewed papers and videos for future Features.
ACKNOWLEDGMENTS
Anna Strimaitis, a Middlebury undergraduate and Biology major, was immensely helpful searching the recent research literature for appropriate videos and preparing the still figures for this Feature.



