Student Learning of Early Embryonic Development via the Utilization of Research Resources from the Nematode Caenorhabditis elegans
Abstract
This study was undertaken to gain insights into undergraduate students' understanding of early embryonic development, specifically, how well they comprehend the concepts of volume constancy, cell lineages, body plan axes, and temporal and spatial dimensionality in development. To study student learning, a curriculum was developed incorporating resources from the Caenorhabditis elegans research community. Students engaged in a preactivity assessment, followed by instructional materials (IMs) emphasizing inquiry-based learning and a postinstruction assessment to gauge their learning. This study, conducted at two research sites with eight and nine students, respectively, shows that before instruction, most students confused embryonic cell cleavage, where total volume is constant, with regular cell division, in which total cell volume doubles. Despite their ability to construct a cell lineage tree, most of the study participants were not aware of its biological significance. All students correctly identified cells of anterior and posterior axis, but not cells of the dorsal and ventral axis. Although the students had no difficulty with the time dimensional aspect of development, most viewed an embryo as spatially two-dimensional rather than three-dimensional. Furthermore, this study indicates that combining authentic research resources with inquiry-based learning benefits student learning of key concepts in embryology.
INTRODUCTION
Developmental biology encompasses one of the most intriguing phenomena in biology—the development of a fully formed organism from a fertilized egg. This developmental process involves complex biological mechanisms spanning an array of disciplines, including anatomy, physiology, biochemistry, genetics, and cell biology (Slack, 2003). Embryonic development involves several key steps, including cleavage of the fertilized zygote, and establishment of body axes. The timing of these processes must be precisely orchestrated because errors in one process can lead to problems in subsequent steps, potentially resulting in lethal developmental failures (Campbell and Reece, 2005). Before the onset of gastrulation, three closely linked developmental processes—embryonic cleavage, the initiation of cell lineages, and axes formation—occur. Embryonic cell division in Caenorhabditis elegans (C. elegans) is characterized by a cleavage process in which, despite an increase in cell numbers, the overall embryo volume stays constant. As the cells divide, they begin to take on characteristics that will ultimately define the variety of body structures apparent in the adult organism. This state of differentiation may be a consequence of several factors including the following: the cell's genealogy, the developmental time at which it arises, its environment, as well as its pattern of gene expression and protein localization. An understanding of the embryo's body plan can be obtained by assembling a lineage tree of the dividing cells within the embryo. The axes of this body plan are spatial entities that develop over time. This constitutes another important component in the study of development—development progresses in space and through time (three spatial dimensions—length, width, and height—plus time = four-dimensional [4D]). As such, development can only be fully understood in a 4D context (Eils and Athale, 2003; Mohler, 1999; Thomas et al., 1996).
Previous reports have identified concepts in early embryonic development, including cell fate determination and three-dimensional (3D) organization (Guidice and Onorato, 2003; Lim, 2003; Moore, 1986; Vignali, 2003), as important for student learning. However, because little research has been undertaken to probe students' understanding of these fundamental concepts, there is limited knowledge of students' conceptual difficulties when learning these key points about early embryonic development. In this study, we developed, and subsequently taught, a curriculum that incorporates resources using a model organism, the nematode C. elegans. Our goal was to gain insight into undergraduate student learning of three fundamental concepts in developmental biology:
(a) Constant Volume during Early Embryo Development. There is an increase in the number of cells yet the overall volume remains constant. | |||||
(b) Cell Lineage and Body Plan Axes. The division of embryonic cells can be described by a cell lineage, which also defines the body plan axes. | |||||
(c) 4D Development. Development proceeds in four dimensions, specifically the three dimensions of space plus the dimension of time. | |||||
This curriculum emphasizes an inquiry-based pedagogic approach (Lu et al., 2007a; Peterson and Jungck, 1988) that enables students to explore biological phenomena using authentic research data.
MATERIALS AND METHODS
Participants
This study was conducted at two research sites in Madison, Wisconsin.
Site 1.
The participants were undergraduate students at the University of Wisconsin–Madison enrolled in the introductory-level summer laboratory course Animal Biology. This class had an enrollment of eight female students, all of whom agreed to participate in the study. Students in this course are required to take the associated Animal Biology lecture before, or concurrent with, the laboratory. This class included students, primarily freshmen, both completing biology majors as well as students from other majors satisfying the biology requirements for their particular programs.
Site 2.
Participants from this research site were undergraduate biology majors taking an intermediate biology course, Organismal Zoology, at Edgewood College, a liberal arts college. The seven female and three male students in the class had completed more than one semester of biology course work before enrolling in this course. All students agreed to participate but one was absent during the study resulting in a total of nine participants from this site.
In both research sites, the first author worked with the course instructor to administer course materials and facilitate student activities. With additional preparation and class time, we envision this unit could be applied to large-enrollment classes because the instructors primarily facilitate student activities rather than lecture.
The Biology behind this Curriculum
Constant Volume During Early Embryo Development.
The early development of many nonplacental embryos is characterized by their cell cleavages, or division, patterns. In most nonembryonic cells, there is a period, after division, in which the daughter cells grow to the size of their mother cell before they divide again. In contrast, embryonic divisions lack the growth phase of the cell division cycle. Thus, the two daughter cells are each half the volume of the mother cell, resulting in an increase in cell number while the size of individual cells becomes smaller. Thus the overall embryo volume remains constant.
Lineage Tree of Cells, Axes of Body Plan, and 4D Development.
The cell lineage describes a cell's predecessors and its successors. With an invariant pattern of cell division, such as occurs in C. elegans, a given cell always appears at a specific time and certain position with respect to its neighboring cells in every embryo (Sulston et al., 1983). In a bilaterally symmetric organism, three body axes are established early on in embryonic development: anterior/posterior (A/P), dorsal/ventral (D/V), and left/right (L/R). As previously described, 4D development refers to the concept that embryos have a spatially 3D structure that changes through time. Figure 1 illustrates constancy in volume, cell lineage, body plan axes, and 4D development during early embryonic development of C. elegans.
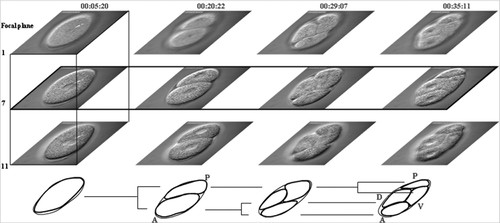
Figure 1. The figure illustrates the concepts of volume constancy, cell lineage, body axes formation, and dimensionality in early cell division of a C. elegans embryo. Images used in the illustration were acquired with differential interference contrast (DIC) microscopy. Images show an embryo developing from a zygote to a 4-cell embryo. Numbers on the top of the figure indicate time in hour:minute:seconds. Four developmental time points are shown (columns). Images in the same row are acquired from the same focal plane (delineated more clearly in the second row, time dimension), with three focal planes (1, 7, and 11) shown per time point (delineated more clearly in the first column, spatial dimension). The illustration at the bottom shows the lineage of the cells and axes of body plan of the embryo. The lineage tree begins with the 1-cell embryo (time 00:05:20). The 1-cell then divides (time 00:20:22) into 2-cell with the larger daughter cell being anterior (A) and smaller one being the posterior (P) axis of the embryo. The anterior cell divides first to generate a 3-cell stage (time 00:29:07). The posterior cell divides soon after, generating a 4-cell stage (time 00:35:11). By this stage, the embryo has established two axes: anterior(A)/posterior(P) and dorsal(D)/ventral(V).
C. elegans Curriculum of this Study
The curriculum used in this study was taught in both research sites as a 3-h self-standing course unit called “C. elegans.” Table 1 includes a brief summary of class activities in the C. elegans unit.
| Preactivity | 1. Construct early embryo development up to the 4-cell stage using Play-Doh and beads. |
| 2. Draw three diagrams to depict relationship of developmental time, volume, and cell numbers. | |
| 3. Observe illustrations of C. elegans early embryo development and explain their observations. | |
| C. elegans IMs | 1. Students were introduced to background information such as cell lineage, axis formation, dimensionality through a combination of illustrations, images, and movies online. |
| 2. Students constructed transparency embryos. | |
| 3. Students drew lineage tree and provided observation and explanation. | |
| 4. Students completed two charts regarding the embryo's biological characteristics in 3D and 4D. | |
| Postactivity | 1. Students presented their 4D transparency embryo and reflected on their learning before and after the C. elegans IMs. |
| 2. Students observed a new dataset of a C. elegans embryo development and described their observation. |
Over the course of this unit, students inquired into biological phenomena encountered in various activities (Lu et al., 2007a). The unit was organized into three sections: 1) a preactivity, 2) the C. elegans Instructional Materials (IMs), and 3) a postactivity. The preactivity was used to determine students' views of early embryo development. During the preactivity, the students were asked to construct a representation of a growing embryo from the 1-cell to 4-cell stage (8-cell stage for Site 2), including nuclei, using Play-Doh and beads. They then completed three graphs to show the relationships between cell number, development time, and embryo volume. These graphs, along with the students' Play-Doh constructions, were used for data triangulation. The students were also given illustrations (Figure 2) of early development of a C. elegans to help determine their views about size and dimensionality. One set of illustrations showed one section (a single focal plane) of the embryo from the 1-cell to 4-cell stage (2D + time) (Figure 2, A–D). The other represented one embryo in space, with 12 sections (12 focal planes, 3D at a single time point) (Figure 2, E–H).
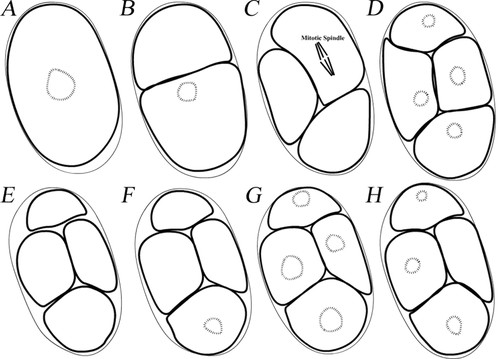
Figure 2. Representations of a C. elegans embryo illustrating the temporal and spatial dimensionality of development. (A–D) Illustrations of a C. elegans embryo from the 1- to 4-cell stage showing changes over time. These illustrations were from one focal plane at the developmental time points of the 1-, 2-, 3- and 4-cell stage. Some cells did not have any genetic material (hashed circles) present due to the depth of this particular focal plane. (E–H) These illustrations were made from the same embryo at the 4-cell stage at the same time point showing differences in the spatial dimension. Twelve focal planes (sections) were used for student observation. Only focal planes of 1 (E), 4 (F), 7 (G), and 11 (H) are shown here as examples. Focal planes of 1 and 11 are closer to the top and bottom, respectively, of the embryo such that genetic material is not visible in all of the cells. Focal plane 7 is close to the center of the embryo, thus the genetic material can be seen in all the cells.
During the instruction, students were given background instruction and relevant terminology regarding C. elegans and the topics they would be studying. Students observed real C. elegans using brightfield microscopes, without DIC optics. Images and computer animations of C. elegans embryos (http://www.wormclassroom.org/cb/cellPolarity.html) were also presented to assist student learning (Lu et al., 2007b). Students were subsequently given additional instruction regarding the cell lineage of C. elegans from an original research publication (Sulston et al., 1983). They then observed a 4D movie (Figure 1) of a C. elegans embryo with the cell lineage color-coded from the 1-cell to the 100-cell stage. This information was presented as 12 movies such that each movie represented one focal plane through time. During movie observation, students were instructed to pay close attention to the specification of body plan axes. They were then asked to use transparencies to construct a series of 3D representations at different time points; thus, they generated a 4D (3D time-lapse) representation, based on a subset (four focal planes, five time points: the meeting of paternal and maternal pronuclei + stages of 1-, 2-, 3- and 4-cell) of the movie they had viewed.
Using this 4D transparency-embryo dataset, students first made a cell lineage tree of one focal plane, with the time labeled. They were then asked to complete two charts based on their observations of the embryo from either 3D (space) or 4D (space and time) views of their transparency-embryo.
During the postactivity, students summarized their results verbally. They then were asked to make observations of a new dataset as well as write down and explain their observations. This new dataset was a movie showing the development of a different embryo. The movie showed an embryo developing up to the 4-cell stage and was compiled in such a way that, for each time point, 6 focal planes were displayed before the movie progressed to the next time point. As a result, the embryo appeared to pulsate, because of differences in cellular organization between the focal planes. Not only was the data displayed differently than the movie presented in the instruction but the embryo in this movie was also oriented differently.
Data Collection and Analysis
Pilot studies were performed before the initiation of this study to improve the presentation and content of the C. elegans curriculum. Because more data were obtained from Site 1, this report focuses on data collected from that site. Supporting and opposing findings from research Site 2 are presented when relevant.
Data Collection.
Data collected from this study included Play-Doh representations that students constructed during the preactivity as well as students' in-class written work, such as drawings, observations, and their 4D transparency constructs. In addition, the class was videotaped, with one camera for each student group, to ensure that conversations could be closely monitored. Although the effect of “being watched” is a known issue in educational research for data collection, it best met the needs of this study to obtain detailed, qualitative insight of student learning. During the postactivity, students' responses to their observation of embryo development on the computer were recorded using SnapZ X (http://www.ambrosiasw.com/utilities/snapzprox/), a software program that digitally recorded conversation and cursor movement.
Data Analysis.
The in-class video camera recordings were converted to QuickTime movies to ease analysis and archiving. The audio from these QuickTime movies was transcribed. Students' in-class written work, drawings, and the SnapZ X recordings were used for data triangulation to ensure the quality of the research findings. Gall et al.'s (2003) “interpretational analysis” approach was used to examine the collected data for emerging themes and patterns regarding student learning of early embryonic development. The coding strategy used for analysis was: 1) an individual group's transcript and its accompanying in-class works were used to identify major themes and patterns of student learning and 2) these identified patterns were compiled into a text document. Steps 1 and 2 were repeated for the remaining groups. The identified learning patterns of all the groups were compared for common patterns. Finally, all groups' original data were reexamined for inconsistent themes among all groups to eliminate idiosyncratic data.
The interpretational data analysis approach was used to identify patterns or trends of student learning in the following major areas: significant concept shifts that students made as a result of the learning activities, alternative concepts that students continued to hold even after the learning activities, and particular areas that students continue to find difficult to understand. When inconsistent patterns were found between the groups, the original data of all groups were further examined to ensure that there were major differences between groups rather than trends that were initially overlooked. The specific learning trends, once identified, were then discussed among the study investigators to ensure that this was really representative of the student learning. During the study, students were asked to work individually on the Play-Doh construction and graph-drawing exercises and in teams of two for the remainder of the curriculum activities. Thus, results for the two exercises are presented as individual data, while subsequent data are presented as group data. Student views of early embryonic development and insight to their conceptual learning presented in the Results section are categorized into 1) constant volume during early embryo development, 2) cell lineage and body plan axes, and 3) 4D development. Within an individual category, data from one, or limited number, of groups that represent the view of the majority are presented.
RESULTS
Constant Volume during Early Embryo Development
Preactivity.
To determine the students' preconception of cell division during early embryonic development, the students were asked to construct a model of an embryo's cell division to the 4-cell stage using Play Doh of different colors. The most common representation (seven of eight students) is shown in Figure 3A.
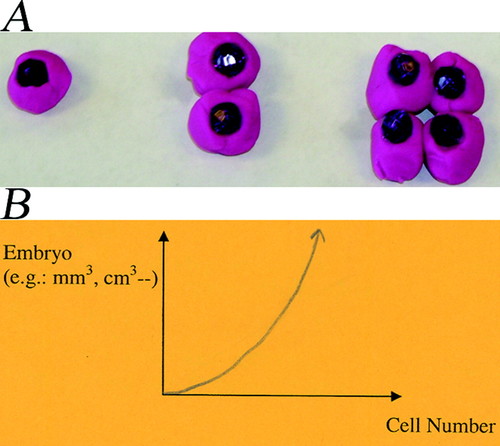
Figure 3. Student preactivity representations of early embryo development. (A) Purple Play-Doh represents cell with beads showing the nuclei. As indicated, the division of cells is accompanied by volume increase of the embryo. (B) A drawing by a student depicting the relationship between embryo volume and cell number showing that they are related exponentially as depicted by their Play-Doh model in A.
Students with the view of Figure 3A claimed that mother and daughter cells are the same in size, as are the two daughter cells. One student, Carly (pseudonyms are used for all students), stated “Because if you start with 1 (cell), it will be bigger by the time you have 4 (cell). Because otherwise, [the] embryo wouldn't get any bigger.” This view of embryo development was also reflected in their plots of the relationship between embryo volume, development time, and cell number (Figure 3B). Similar results were obtained from research Site 2 where, during the preactivity, only one of the nine students viewed an embryo as remaining constant in volume during early development. Thus, the majority of students believed that the embryo increased in volume during early development.
Students then were instructed to observe illustrations that accurately depicted C. elegans embryo development from the 1-cell to 4-cell stage (Figure 2, A–D). When the students observed the constancy of volume in the early C. elegans embryo they reevaluated their own concept and identified possible ways to explain this phenomenon, with eggshell confinement being the most common explanation.
C. elegans Instructional Materials and Postactivity.
Students viewed movies and animations of C. elegans embryos dividing and developing to show that the embryo remained constant in size while cell number increased. During the postactivity, the majority of students reiterated that the restriction of the eggshell was the reason for the constant embryo volume observation. Students Kathy and Laney described that the volume of the C. elegans embryo remained constant because it is confined by the eggshell. When asked if a human embryo increases in size, Kathy replied “it is [getting] bigger.” Thus, even after the IMs, students' views of embryo volume remained consistent with their initial idea that an embryo always increases in size over time if not otherwise restricted by some physical barrier such as the eggshell. Many students were of the opinion that the confinement of the eggshell led to the constant volume observed in the C. elegans embryo. They seemed content with the idea that this explanation would indicate there are substantial differences in constant versus increasing volume between shelled and nonshelled embryos during early development. Table 2 is a summary of student views on constant volume in early development over the course of the C. elegans unit.
| Preactivity | 1. Majority of the students viewed cleavage as accompanied by embryo growth. |
| 2. One student at each research site viewed that an embryo stayed constant in volume. | |
| C. elegans IMs | 1. Majority of the students shifted to constant volume in shelled embryo, but this was not generalized to nonshelled embryos. |
| Postactivity | 1. The majority kept the same view they had during the C. elegans IMs. |
Cell Lineage and Body Plan Axes
Preactivity.
Although most students stated that an increase in cell number was the result of cell division, none of the students identified the relationship between mother-daughter cells or the establishment of body plan axes in either their Play Doh representations or their observations of the illustrations of the C. elegans embryo (Figure 2). Similar results were obtained from research Site 2: none of the students specified the concepts of cell lineage or body plan axes.
C. elegans IM's
To introduce students to the concept of cell lineage and axes of body plan, the complete C. elegans cell lineage described by Sulston et al. (1983) was discussed. Several movies (of a 4D dataset) of C. elegans early development were observed. In these movies the lineage of cells was color-coded according to Sulston's naming convention. In addition, students discussed axis formation while they observed movies of live embryos as well as still images of embryos at different cell division stages (http://www.wormclassroom.org/modules/celllineage/). They were then asked to draw a cell lineage tree, accompanied by time notations using traced transparency of one focal plane. An example lineage is shown in Figure 4A. The students demonstrated an understanding 1) that the process of mitosis generated two cells from one cell, 2) that an asynchronous division from the 2-cell stage temporarily generated a 3-cell embryo, and 3) of how to construct a cell lineage tree that included all cells from the 1- to 4-cell stages. However, when questioned (three of four groups were asked to do so), none of the students were able to correctly associate the lineage of cells diagramed in their trees and actual images from the 2- to 4-cell stages (Figure 4). Students did not identify the significance of the predecessor/successor relationship in the lineage of cells, which required them to closely monitor and determine: 1) which cell divided, 2) what the two daughter cells were, and 3) where the two daughter cells resided.
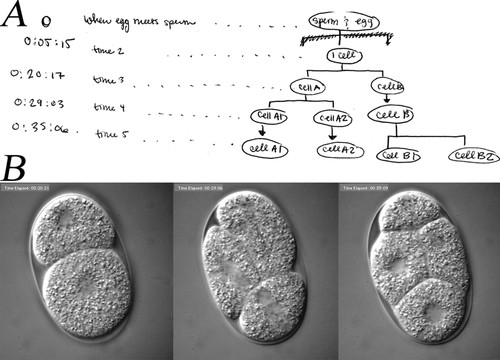
Figure 4. Example of lineage tree of cells. (A) Student diagram of a lineage tree. In this drawing, a fertilized egg became a 1-cell embryo, the 1-cell then divided into cells A and B. Cell A then divided earlier than cell B into cells A1 and A2. Soon, the cell B divided into cells B1 and B2. (B) These are DIC images of a C. elegans embryo from the 2-, 3- to 4-cell stage. These images were used for students to identify relation of cells shown in their lineage tree.
A shift in thinking arose when students were unable to match their lineage tree to images of actual embryo development. Those students who had difficulty determining the relationship between predecessors and successors, after suggestion or on their own, reviewed the movies of embryo development. On this additional view, and by paying close attention to the divisions, the students were able to identify a connection between their lineage tree diagram and the cell pedigree in actual development.
Postactivity.
During presentations and reflections, students explicitly followed and described the lineage of cells from a 1-cell to the 2- then 3-cell stage, with the larger daughter cell subsequently dividing earlier than the smaller daughter cell. Even when a different embryo dataset was used all students explicitly and correctly traced the cell lineage relationship. For instance, one student commented, “that guy (pointed to the bigger cell at the 2-cell stage) is going to split first.”
At this point in the activity, all students noted the existence of the A/P axis and associated the anterior end with the larger cell at the 2-cell stage without difficulty. In addition, by tracking a 2-cell embryo as it cleaves into three and then four cells, three groups of students viewed the developing embryo as establishing its dorsal and ventral sides. However, none of the students were able to correctly and confidently associate dorsal and ventral with the correct cell lineage at the 4-cell stage. Dana, during her presentation, stated “… and we don't know which one is dorsal and which one is ventral. We can tell anterior and posterior, but these (D/V) we don't know.” Such difficulty was further evident during their observations of the movie showing a different developing embryo. This difficulty in associating the D/V axis with the corresponding cells of the embryo was also observed at research Site 2 where students were able to follow the lineage of cells to the 4-cell stage and name the cells correctly, but had difficulty matching the cells to the correct axes. Table 3 includes a summary of student views on the concepts of lineage tree and body plan axes formation.
| Preactivity | 1. None expressed the view of lineage tree or body plan axes. |
| C. elegans IMs | 1. All were able to construct a family tree of cells. |
| 2. When questioned, none were able to identify the cells' pedigree correctly. | |
| 3. After observing movies of a live C. elegans embryo development, all noted the significance of cell lineage relationships. | |
| 4. All were able to identify anterior/posterior axis correctly, but not the dorsal/ventral axis. | |
| Postactivity | 1. All paid explicit attention to the lineage of cells. |
| 2. Majority of the students had difficulty identifying corresponding cells to the axis of dorsal and ventral. |
4D Development
Preactivity.
From the Play-Doh construction, students did not mention dimensionality, even though their models had inherent 3D dimensionality because of the use of Play Doh. When observing the illustrations shown in Figure 2, A–D, all students found it puzzling that in Figure 2, B and C, some cells did not exhibit genetic materials. None of the students viewed these 2D illustrations as representing one section of a 3D embryo at different time points. Most students (three groups) did not have an alternative concept to explain these illustrations. Some students speculated that cells without genetic material were preparing for, or had just completed, division. This idea aligned nicely with the biological concept that the nuclear envelope disappeared and reappeared before and after a cell division. However, the concept of cells dividing cannot fully explain the illustrations: 1) how the upper cell in Figure 2B (a cell without visible genetic materials) divided into the two lower cells in Figure 2C (the two cells without visible genetic materials) and 2) a mitotic spindle was not present in the dividing cell.
During observation of illustrations in Figure 2, E–H, half of the students remained uncertain as to why some cells had a nucleus and some did not. One student, Kathy, remarked that “[C. elegans] some were born without DNA anywhere.” The remaining students (two groups) interpreted these illustrations with a 3D view. One of the teams, Eva and Alicia, despite the fact that these illustrations were from one embryo at the same time point, interpreted these 12 illustrations as being 12 sections from different embryos (12 embryos possibly) all at the same time point. They claimed that the nuclei or chromosomes may have been floating in the other half of the cross section, explaining why some cells have no visible genetic material.
C. elegans IMs.
Students viewed a 4D dataset (12 movies) of a developing C. elegans embryo. Each of the 12 movies showed one focal plane of the embryo developing over time. The students also made transparency tracings of different focal planes of an embryo (spatial dimension) and at different cell stages (temporal dimension) (Figure 5). One group of students, Dana and Carly, exhibited a more complete understanding of the 3D embryo than did the remaining students at the initial point of the instruction. While working on the chart regarding 3D (four focal planes at a time) properties of an embryo, they examined their four transparencies as a stack (Figure 5E) to form a 3D representation of the embryo. They remarked that the embryo was spherical. For instance, when examining the size of an embryo across different focal planes, Carly argued that “… well, if you think it is like the way it is [gesturing oval embryo]. Surely, it doesn't seem like it would be like uniform.” Dana responded that “… these two [middle focal planes] definitely are the biggest.”
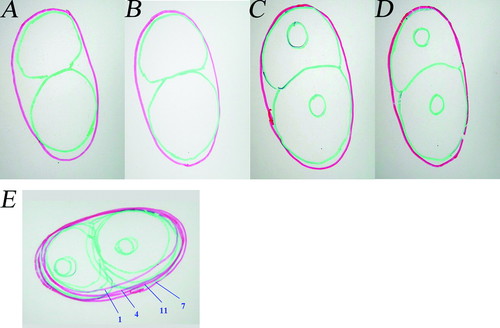
Figure 5. Example 3D transparency-embryo at the 2-cell embryonic stage. Green indicates the embryo's cell membrane, red indicates the eggshell. (A-D) individual tracing of focal planes 1, 4, 7, and 11. (E) A stack of transparency tracings of four focal planes that formed a 3D looking transparency-embryo. The number of individual focal planes was labeled. The focal planes were labeled according to the size of the individual eggshell (from left to right, smallest to biggest: 1, 4, 11, 7).
The remaining students (who had a less complete view of the dimensionality concept), when examining transparencies of four focal plane at a single time point, made the following observations: 1) genetic material could be seen always, 2) the eggshell was the same size, or 3) the embryo was the same size. They viewed either eggshell or the embryo as being uniform in size throughout space. For instance, Anna and Carrie claimed that the size of the eggshell in all focal planes of a 3D embryo was the same. Carrie thought that she may have traced the eggshell wrong when she noted there was a discrepancy between the sizes of the eggshell between two focal planes. It is clear at this point that, like most other students, Anna and Carrie viewed the four 2D transparencies as a series of 2D representations. They questioned their tracing ability when inconsistencies arose between their drawings of transparencies and their view of the embryo. They did not seem to conceptually grasp the concept of dimensionality when viewing the embryo at a single time point.
A shift in thinking occurred after they were instructed to stack all four focal planes (1, 4, 7, 11) to create a transparent 3D embryo (Figure 5E). While the interviewer was working with Eva and Alicia, Eva observed the stacked four transparencies and shouted “Oh, holy cleverness! Ok, that is certainly growing [change in size between focal planes].” Students seemed to easily grasp the concept of the time dimension in the development of an embryo. They were able to arrange the series of biological events from a zygote to the 2-, 3-, and 4-cell stage using their traced transparency-embryo.
Postactivity.
During their presentations and reflections, all students viewed embryo development in space and time. They understood that genetic material was not present in all focal planes at all times. They viewed the embryo to be spherical in shape with varying properties, such as the size of eggshell, in different focal planes. One student, Kathy, who had previously remarked that “some were born without DNA anywhere” stated that “basically what we found was the cell started dividing, and one thing we noticed was like chromosome seems to kind of reappear and disappear especially. … It all depends; it is not like it doesn't have a chromosome. It just depends on what planes you are looking at.”
During observation of a different embryo's early divisions, in which the embryo movie was presented as a series of six focal planes before changing to the next time point, the students' first reaction to the movie was “why is it pulsating?” After paying close attention to the movie, they were able to ascertain that the movie was going through different focal planes as well as moving through different times in development. One student, Dana, noted “… the nuclei were at times really apparent and at other times were not even visible and everything in between (ex [for example]: it depends on what cross-section you are looking at whether you can see the nuclei or not.).” They were able to apply their expanded 4D view to the observation of a movie of a different developing embryo and describe its changes over space and time. Table 4 summarizes student views on the concepts of dimensionality over the course of the C. elegans unit.
| Preactivity | 1. All understood time as a dimension in development. |
| 2. None mentioned spatial dimension. | |
| C. elegans IMs | 1. Students with more complete spatial 3D view: Viewed biological properties of the embryo as not uniform in all focal planes. |
| 2. Students with less complete spatial 3D view: Viewed biological properties of the embryo uniformally across to all focal planes. By stacking transparencies of all focal planes together, they could view the embryo as 3D. | |
| Postactivity | 1. All viewed the development of an embryo in space and time. |
DISCUSSION
There is limited research on students' understanding of early embryonic development. This study was intended to address this in part by providing some key insight on student learning and understanding of the important biological process of early cell division. Three major biological concepts were the focus of the study: 1) an embryo can undergo cell division without overall increase in volume, 2) cell division occurs in a patterned lineage, leading to a clearly defined body plan axes, and 3) there is both space and time dimensionality to development. The study showed that students were unable to generalize the concept of constant volume in the C. elegans embryo to embryos of other organisms mainly because the students focused on the C. elegans eggshell as being the most likely reason behind this observation. The utilization of C. elegans research resources such as movies of a real C. elegans embryo development allowed students to expand their view of cell lineage tree to include the biological significance of predecessor and successor relationships. The 4D transparency embryo model allowed students to visualize an embryo in the three spatial dimensions at a single point in time. The process of learning by inquiry with this model helped students shift their view of the embryo from 2D to 3D over time.
Before the instruction, most students were of the opinion that, in early development, an embryo increased in volume because daughter and mother cells are the same size. After the instruction, although most students shifted their view to one in which the embryo volume remained constant, they were uncertain whether embryos without an eggshell stay constant in volume during early development, with many students concluding that the eggshell restricted growth, preventing an increase in volume. Their views that embryos increased in volume seemed to demonstrate an incomplete understanding of how embryonic cleavage proceeds in free living (i.e., nonplacental and preimplantation) embryos. Instead, their views of early embryo division seemed to be consistent with their understanding of mitosis of nonembryonic cells in which each daughter cell grows to the same size as the mother cell before the subsequent division. The volume constancy in nonplacental eggs has also been observed in placental embryo development (e.g., mice, before preimplantation [Aiken et al., 2004]). In the case of early development, an embryo contains only limited amount of energy and resources, such as proteins, to undergo development. Increasing cell numbers without growth may provide an effective strategy by conserving these resources, thus ensuring development and future propagation. Therefore, as an example of the broader concept of resource distribution and survival strategies, as well as a crucial concept in itself, it is important for students to understand the significance of cleavage without increase in overall volume. For future studies, the use of shelled and nonshelled embryos (http://www.visembryo.com/baby/) would be ideal because they would provide students with the opportunity to inquire into early embryo development and help to further overcome the alternative conception that early embryonic cleavage must always be accompanied by growth. For instance, movies of a mouse embryo (mammalian, nonshelled) development to the blastocyst stage would provide students the opportunity to compare and contrast development of different embryos. In addition, it allows students to consider the alternative concept that early development of an embryo does not involve a volume increase.
By tracing different stages of a cleaving embryo, a lineage tree of cells can be derived that includes information about the organism's body plan axes. None of the students expressed the view of lineage tree or body plan axes during the preactivity. Possible explanations are 1) students might not have appreciated the importance of the mother-daughter cell relationship and its relevance to axes formation at such early stage in development, or 2) they were not specifically prompted to make such observations. Early in the instruction, students expressed the view that a cell lineage tree has an origin and they correctly identified that origin. They understood the tree expanded by cell division. However, a majority of the students were unable to associate the pedigree relation between a lineage tree and the actual embryo development. Hence, the significance of the lineage tree in embryo development was overlooked, an oversight that may have further impeded their learning of body-axis specification. Toward the end of the instruction, student learning of lineage relation was enhanced by their tracking of cells in movies of C. elegans ' early development. After the instruction, students identified the traceable link between successor and predecessor cell generations, which provided the students with an enhanced understanding of cell role and propagation.
Our findings imply that students initially did not view a lineage map of cells as having biological significance. The significance of cell lineage is derived from tracing a cell's predecessors and successors, as well as locating the two daughter cells spatially. A traditional tree diagram, such as that usually found in a textbook, does not allow for tracking such dynamic relationships. The presentation of movies of real embryo development with a corresponding color-coded cell lineage provided students with an opportunity to explore, trace, and associate developmental significance to the cleaving cells. The movie for student observation included characteristics of 1) asymmetric cleavage from the 1- to 2-cell stage, 2) asynchronous cell division from the 2- to 4-cell stage, and 3) color-coded cell lineages of real embryo development. The significance of these individual characteristics in student understanding of cell lineage remains to be determined. However, our data certainly suggest that students may learn about the cell lineage more effectively when using movies of the processes as compared with using a lineage tree map alone.
In C. elegans, the identification of body axes requires matching and memorizing axes to their corresponding cells. During the instruction, students began to make the connection between cell lineage and the establishment of body axes in the organism. Toward the end of the unit, all students were able to identify the anterior and posterior of a C. elegans embryo. However, they still did not find it equally intuitive to match the dorsal/ventral axis with the cells that gave rise to that axis. Possible explanations are: 1) There are twice as many cells in the 4-cell stage than the 2-cell stage, making the embryo organization more complicated for students to comprehend; 2) The size difference between the two cells in the 2-cell stage was more pronounced than the cell size and shape difference present at the 4-cell stage; 3) It required tracing cell lineages as well as memorization and matching more cells and axes at the 4-cell stage.
The size differences observed in the early development of C. elegans did help students in A/P axis identification. In addition, the observed inability of students to properly match cells with D/V axis does not imply that students did not understand the concept of body-axis formation in early development. Instead, it may simply be that students matched the specific relation between cells and axes names incorrectly because of the instructional approach that did not require them to memorize such relation.
All developmental events, by their very nature, take place both in space and time, thus embryonic development can only be properly understood within a 4D perspective. Before the instruction, students seemed to view embryo development as a 2D entity changing in time. This view was consistent with their prior experience of 2D representations of cells, such as those typically found in textbooks, in which all cell components are shown, even though their true representation requires spatial dimensions. Possibly as a result of this limitation of traditional learning materials, students in this study attempted to explain illustrations from a 3D embryo solely from a 2D perspective. Some students quickly grasped the concept of dimensionality of an embryo during the preactivity. During the instruction, the remaining students, who initially had a less complete 3D understanding, started to include spatial dimensionality in viewing the embryo. The utilization of a transparency embryo model seemed to help students expand their view of embryo development from two dimensions to three dimensions in time. Adopting such a 4D view subsequently allowed the students to understand the developmental changes occurring in a movie depicting a different embryo developing.
These observations of student learning indicate that the general concept of three-dimensionality was not new to the majority of students, it was just in the context of embryo development that the students struggled with the dimensionality concept. Possible explanations for students not viewing the embryo three dimensionally could be either that the students were not used to thinking about biological events in a 3D context or, perhaps, the connection between biological events and three-dimensionality was not explicitly made during the students' prior instruction.
The C. elegans unit was developed to gain insight into student views of some of the important biological concepts in early embryonic development. This unit could serve as a basis for additional teaching and learning opportunities. For instance, the green fluorescent protein (GFP) derived from the jellyfish Aequorea victoria is often fused with a gene of interest to study a gene's phenotypic expression. Resources such as GFP movies and genetic data have great utility for teaching and learning difficult concepts, such as how body-axis polarity is achieved during early development. This study focused on the novel use of authentic research data from the nematode C. elegans to teach fundamental concepts in embryology and developmental biology with positive impact demonstrated. In addition, as neither research data nor embryology is taught regularly in most college Introductory Biology courses, it could be argued that the best way to demonstrate the merits of using research data is to apply it to a more commonly taught topic, such as one based on biodiversity. While often treated as an advanced concept in biology, it is our opinion that embryology has great promise as a focal point for an introductory curriculum because the subject matter is accessible to students, it engages their interest, and helps explain biological processes related to other more traditional topic offerings, such as evolution.
Although it was beyond the focus of this study to carefully examine and evaluate the advantages as well as the possible disadvantages of such a curriculum as compared with the more conventional textbook-based inquiry, it is our hope that future studies will do so. As well, future studies into model organism–based curricula should examine the possible drawbacks of this instructional approach such as species-specific characteristics. However, because of the abundance of research resources available for model organisms, such as the nematode C. elegans and the fruitfly D. melanogaster, they hold great educational promise to serve as aids for teaching fundamental concepts, as has been demonstrated in this study.
ACKNOWLEDGMENTS
The authors thank the instructors, Dr. Gale Oakes, Devin Biggs, and Dr. Francie Rowe, and undergraduate students in the 2005 Zoology 102 class at the University of Wisconsin–Madison and Organismal Zoology 352 class at Edgewood College for their participation in this study. We also acknowledge the contributions and input of Dominika Bienkowska and Kristin Riching at the University of Wisconsin–Madison. This work was supported in part by National Science Foundation DBI-9983114 and National Institutes of Health National Institute of Biomedical Imaging and BioEngineering grant no. R01-EB000184.