Alternation of Generations and Experimental Design: A Guided-Inquiry Lab Exploring the Nature of the her1 Developmental Mutant of Ceratopteris richardii (C-Fern)
Abstract
Inquiry-based labs have been shown to greatly increase student participation and learning within the biological sciences. One challenge is to develop effective lab exercises within the constraints of large introductory labs. We have designed a lab for first-year biology majors to address two primary goals: to provide effective learning of the unique aspects of the plant life cycle and to gain a practical knowledge of experimental design. An additional goal was to engage students regardless of their biology background. In our experience, plant biology, and the plant life cycle in particular, present a pedagogical challenge because of negative student attitudes and lack of experience with this topic. This lab uses the fern Ceratopteris richardii (C-Fern), a model system for teaching and research that is particularly useful for illustrating alternation of generations. This lab does not simply present the stages of the life cycle; it also uses knowledge of alternation of generations as a starting point for characterizing the her1 mutation that affects gametophyte sexual development. Students develop hypotheses, arrive at an appropriate experimental design, and carry out a guided inquiry on the mechanism underlying the her1 mutation. Quantitative assessment of student learning and attitudes demonstrate that this lab achieves the desired goals.
INTRODUCTION
Over the past decade, there has been a move to replace traditional expository lab exercises with active, interdisciplinary labs that promote student involvement in the process of discovery (National Research Council [NRC], 2003; Handelsman et al., 2004). Challenging students to use the scientific process to solve realistic problems increases participation and generates a sense of ownership in learning, improves comprehension and retention of content, and helps students to develop critical-thinking and research skills (Domin, 1999; Russell and French, 2002; Chaplin, 2003; NRC, 2003; Luckie et al., 2004; Howard and Miskowski, 2005; Casem, 2006). One obstacle to the adoption of inquiry-based learning is the availability of tested procedures, particularly those that are appropriate for large introductory lab classes. Another challenge that we have encountered is the widely differing experiences that our students bring to the classroom; students who have had intensive biology classes in high school may find a lab to be too simple, whereas those with only a basic biology background may find the same lab to be too challenging. We have addressed these challenges through the design of a simple, effective guided-inquiry laboratory activity for first-year biology majors, in which students characterize the her1 mutant strain of the fern Ceratopteris richardii (known by the trademarked name C-Fern; www.c-fern.org), which has altered sexual development. This exercise integrates molecular and physiological principles within the context of the plant life cycle, building on material from earlier courses, and it helps students learn important concepts related to the plant life cycle and experimental design.
Students are typically less interested in plant biology than other biological topics, because they have had little exposure to plants in previous classes (Uno, 1994), and they are unaware of the unique attributes and importance of plants to their everyday lives (Wandersee and Schussler, 1999). Teaching of plant biology, particularly the plant life cycle (Hickok et al., 1998), presents a unique pedagogical challenge that requires instructors to engage students with hands-on laboratory exercises. Here we describe a guided-inquiry module for teaching alternation of generations, the defining characteristic of the plant life cycle. The key distinction between the plant and animal life cycle is the timing of meiosis, gamete formation, and fertilization. Meiosis in plants results in the formation of haploid spores. Spores develop into a multicellular-haploid stage (the gametophyte) in which gametes are produced by mitosis. Fertilization of the egg within the female gametophyte results in the first cell of the sporophyte (diploid) generation, the zygote.
Ferns (phylum Pteridophyta), like all seedless vascular plants, have independent gametophyte and sporophyte stages; therefore, they are particularly useful for teaching alternation of generations. C-Fern (www.c-fern.org) is a tropical homosporous fern developed by the National Science Foundation and the University of Tennessee as a model organism for teaching (Renzaglia and Warne, 1995; Hickok et al., 1998; Hickok and Warne, 2004) and research (Chasan, 1992; Cooke et al., 1995; Hickok et al., 1995; Banks, 1999). C-Fern is one of the 10 research systems developed as part of the Research Link 2000 program of the Council on Undergraduate Education (http://www.cur.org/reslink2000.html). C-Fern is particularly useful for introductory biology labs because of the ability to observe and manipulate the developmental stages within the life cycle. Using simple and inexpensive procedures, spores will develop within 10–12 d into morphologically distinct male and hermaphroditic gametophytes (Figure 1). Fertilization is triggered when a drop of water causes the release of sperm that swim chemotactically to the egg within the archegonium, with the entire process being visible under a microscope. The resulting sporophyte emerges within 2 wk and forms spores, completing the life cycle within 90 d. The rapid life cycle of C-Fern makes it a useful genetic system, and it has allowed for the generation of several mutant lines with altered development and physiology, which are amenable to inquiry-based lab exercises (Hickok and Warne, 2004).
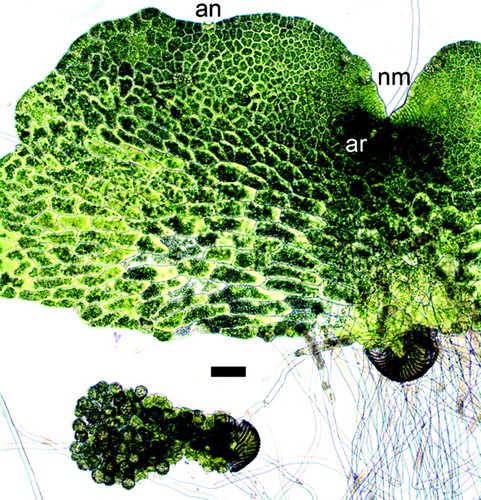
Figure 1. Representative image of a hermaphroditic and a male gametophyte after 11 d of culture. The hermaphroditic prothallus grows to be much larger than the male because of continued cell divisions in the notch meristem (nm). The hermaphrodite forms egg-producing archegonia (ar) below the nm and sperm-producing antheridia (an) to the periphery of the prothallus. The male lacks a meristem and undergoes differentiation of nearly all of its cells to form antheridia. Bar, 0.1 mm.
In this study, we used the her1 mutant in which sexual development is altered such that only hermaphrodite, but not male, gametophytes are formed (Banks et al., 1993; Banks, 1994). In a wild-type population, the earliest-germinating spores develop as hermaphrodites that produce the pheromone antheridiogen (referred to as ACE for antheridiogen of Ceratopteris), which promotes male development in less mature gametophytes. The developing gametophytes are maximally sensitive to ACE 4–6 d after spore germination, and they require continuous exposure to ACE during this period to become sexually determined as males; gametophytes not exposed to ACE during this period develop as hermaphrodites (Banks et al., 1993). ACE blocks cell division and promotes antheridial formation from nearly every cell of the male prothallus; in contrast, hermaphrodites retain an active meristem that results in a much larger, heart-shaped prothallus possessing both antheridia and archegonia (Figure 1). The proportion of males increases with increased population density due to higher concentrations of ACE.
Here we describe a 2-wk lab exercise in which students make observations on sexual development in wild-type and her1 cultures at various population densities, propose hypotheses regarding the nature of the her1 mutant, and generate an experimental design containing the appropriate variables and controls. The students ultimately test their hypotheses through guided inquiry (Domin, 1999) as a whole class and analyze class data to determine whether the results support their hypotheses. This exercise is incorporated into a laboratory module focused on the developmental, structural, and physiological diversity of plants.
LAB PROCEDURE
Curricular Details
The procedure described was incorporated into the laboratory portion of Biology 206, Organismal Biology, which is the second of a four-part core course sequence required of biology majors at Bucknell University. This course typically has an enrollment of 160–180 students, who are primarily in their second semester and are majoring, or considering a major, in biology, cell biology and biochemistry, animal behavior, neuroscience, or biomedical engineering. The students are typically divided into two or three lecture sections and 11 lab sections. The lecture and lab components are highly integrated, and they are focused on diversity, structure, and physiology of animals, plants, and to a lesser degree fungi, protists, and prokaryotes. The labs are designed to reinforce concepts previously covered in lecture, to make connections to material from other courses, to provide inquiry-based experiences, and to allow students a chance to discuss the material in a small class setting. Most students in Biology 206 have taken Biology 205, Introduction to Cells and Molecules; therefore, many of our labs build on this background by focusing on the cellular and molecular mechanisms that underlie organismal processes. The lab courses are each taught by full-time instructors or tenure-track faculty with the assistance of undergraduate teaching assistants. Each faculty member who teaches a lecture section also teaches at least one lab section.
Lab Procedure Overview
This exercise is part of a 2-wk laboratory module focused on the diversity of seedless plants. Students are introduced to the developmental, structural, and physiological characteristics of mosses (phylum Bryophyta), liverworts (phylum Hepaticophyta), clubmosses (phylum Lycophyta), and ferns (phylum Pteridophyta). We provide representative living specimens of gametophytic stages, sporophytic stages, or both of each of these phyla along with detailed study sheets. This diversity survey, which includes the sporophyte of C. richardii, allows students to see first-hand the relationship between the gametophyte and sporophyte in the plant life cycle.
In both lecture and in the laboratory manual, the generalized plant life cycle, the specific stages of the fern life cycle, and the role of ACE in sexual development are covered in preparation for this guided-inquiry lab exercise (Supplemental Material A). This information provides the essential background for the students to develop hypotheses regarding the specific mutation of the her1 strain of C-Fern. Additionally, the lab manual introduces students to the scientific process involving the idealized stages of 1) making an observation, 2) generating questions, 3) developing a testable hypothesis, and 4) designing and carrying out an experiment. The lab manual also introduces the use of independent, dependent, and control variables; treatment groups; control groups; and replicates in experimental design. Students are encouraged to read the lab manual before class; however, the instructors reiterate this content through a short lecture at the beginning of the lab period.
The guided-inquiry lab exercise is divided into 2 wk; during the first week, students make observations on the sexual development of wild-type and her1 gametophytes at various culture densities, they develop a hypothesis regarding the mechanism by which her1 has altered sexual development, and they set up an experiment to test this hypothesis. During the second week, the data are collected and analyzed in preparation for a written report to be prepared by each student for the following week. Class discussion plays an important role throughout the processes of making the initial observations, developing the hypotheses, designing the experiment, and analyzing the data.
Observing her1 and Wild-Type Cultures and Generating an Appropriate Question
Students were provided with sexually mature gametophyte cultures (10–12 d old) of wild type and her1. Both strains were plated at densities of approximately 150, 75, 38, 19, and 9 gametophytes per plate on 60- × 15-mm culture dishes with solid C-Fern medium (Supplemental Material B, see lab preparation notes for culture materials and methods). After an introduction to the morphological differences between male and hermaphroditic gametophytes, each student was required to demonstrate that he or she was able to correctly identify the sex of the gametophytes. Each student determined the ratio of males to total gametophytes in two plates (Supplemental Material A, see lab manual for detailed procedure). The data from all lab sections were combined, and they were provided to the students online as raw data in an Excel (Microsoft, Redmond, WA) file. Each student calculated the average percentage of males for each culture condition and graphed these data as a part of the lab report (Supplemental Material A, see lab manual for lab report content and format). In each of the 5 yr that this lab has been taught, the students have demonstrated a consistent effect of population density on the proportion of males for wild-type gametophytes, whereas the her1 gametophytes do not form males at any density (Figure 2). In each lab section, a rough plot of these data was constructed to generate class discussion. Invariably this discussion led students to formulate questions related to the specific impairment that prevents the her1 strain from forming male gametophytes.
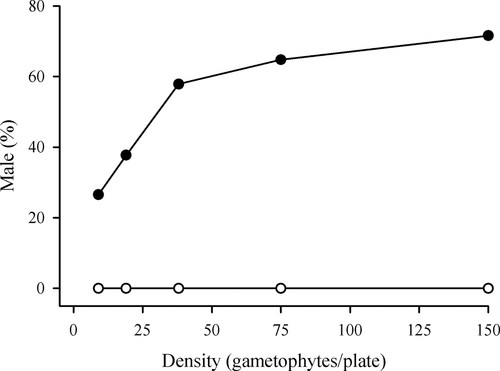
Figure 2. Effect of gametophyte density on the percentage of males within the population at densities of approximately 150, 75, 38, 19, and 9 gametophytes per 60-mm-diameter plate for wild-type (closed circles) and her1 (open circles) strains. The data represent the average of the plates from all 11 lab sections (12–18 replicates per treatment).
Developing a Hypothesis
Students formed small groups for approximately 5 min to generate hypotheses as to the specific mechanism that leads to the mutant phenotype observed in her1, taking into account the known role of ACE in promoting male sexual development. Students were called upon to contribute their hypotheses to a class discussion. The students consistently and correctly proposed that the her1 mutation must lie in genes involved in either 1) the ACE signal transduction pathway or 2) the synthesis of ACE. This led to the development of two competing testable (falsifiable) hypotheses: I) her1 is unable to respond to ACE and II) her1 is unable to produce ACE.
Designing the Experiment
Students reformed their groups to design an experiment to test each of the two hypotheses. To assist in developing a feasible plan, a list of available materials was given, which included immature cultures of both wild-type and her1 and filtrate from 10- to 12-d-old liquid cultures of both wild-type and her1 at full strength (100%) and dilutions of 33, 11, 3.7, and 1.2%. Students were instructed that their experimental plan should describe the independent and dependent variables and the appropriate positive and negative controls.
Through the discussion, the process of student discovery was revealed. Most students first translated their hypotheses into “known” and “unknown” elements. The known elements were that wild-type gametophytes would respond to ACE by increasing the percentage of males and that wild-type filtrate contains ACE. The unknown elements were whether her1 would respond to the addition of ACE and whether the her1 filtrate contained ACE. Students then used the known and unknown elements to develop specific testable predictions. Students consistently determined that hypothesis I (her1 is unable to respond to ACE) would be falsified if addition of wild-type filtrate caused males to form in her1 cultures in a similar manner as in wild-type cultures. Hypothesis II (her1 is unable to produce ACE) would be falsified if her1 filtrate promoted an increase in the percentage of males in wild-type cultures. Class discussion was used to generate a table outlining the independent and dependent variables and positive and negative controls to be used to test the competing hypotheses (Table 1).
| Hypothesis | I: her1 cannot respond to ACE | II: her1 cannot produce ACE |
|---|---|---|
| Independent variable | Concentration of wild-type filtrate added to her1 cultures | Concentration of her1 filtrate added to wild-type cultures |
| Dependent variable | Percentage of males in her1 cultures | Percentage of males in wild-type cultures |
| Positive control | Wild-type filtrate added to wild-type cultures | Wild-type filtrate added to wild-type cultures |
| Negative control | No filtrate added to her1 cultures | No filtrate added to wild-type cultures |
Carrying Out the Experiment
To carry out a feasible experiment with 160 students, given limited time, bench space, and supplies, it was necessary to standardize the experimental design for each of the lab sections. The instructors explained that the experimental conditions have been optimized through several years of teaching this lab. The optimal conditions for response to culture filtrate involve the use of cultures at a density of 38 gametophytes per plate and an age of 3–4 d, which represents a development stage before the period of maximal responsiveness to ACE (Banks et al., 1993). The experimental design included 17 treatment and control plates in total (Table 2). Each group of students was assigned five treatment and control conditions so that each condition was replicated at least 5 times across all lab sections. The students removed the plates from their culture chambers, labeled each plate with the appropriate treatment or control and their initials, and added 200 μl of filtrate to the center of each plate and rotated the plate so that the entire surface was exposed to the filtrate; 200 μl of distilled water was added to those plates that received no filtrate. The plates were placed back in the culture chambers for an additional 7 d at which time each group of students determined the percentage of males for their assigned plates.
| Gametophyte culture | Filtrate conc. (%) and origin | |||||
|---|---|---|---|---|---|---|
| her1 | 100 WT | 33 WT | 11 WT | 3.7 WT | 1.2 WT | None |
| Wild type | 100 H | 33 H | 11 H | 3.7 H | 1.2 H | None |
| Wild type | 100 WT | 33 WT | 11 WT | 3.7 WT | 1.2 WT | |
Data Analysis and Lab Report
The data from all of the students were pooled, and they were made available online as an Excel file. Students were asked to average percentage of males for each condition used and to generate a line graph of these data as part of their lab report (Figure 3). In the 5 yr that we have taught this lab, we have consistently seen the formation of an equal or greater percentage of males when wild-type gametophytes are treated with her1 filtrate than when treated with an equivalent concentration of wild-type filtrate, and we have never observed any production of males in her1 cultures at any concentration of wild-type filtrate. These data are consistent with hypothesis I and falsify hypothesis II, suggesting that her1 is able to produce, but not respond to ACE. This interpretation represents the current understanding in the literature (Banks et al., 1993) and it was consistently described correctly by students in their lab reports.
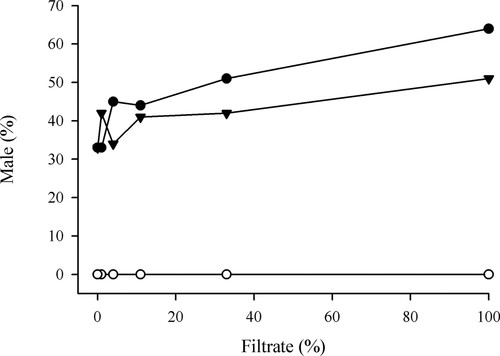
Figure 3. Effect of the concentration of added conditioned filtrate (as a source of ACE) on the percentage of males within the population at a density of approximately 38 gametophytes per 60-mm- diameter plate. Shown are wild-type gametophytes treated with filtrate derived from her1 cultures (closed circles), wild-type treated with wild-type filtrate (closed triangles), and her1 treated with wild-type filtrate (open circles). The data represent the average of the plates from all 11 lab sections (at least five replicates per treatment).
The lab report was composed of an abstract, introduction, materials and methods, results, discussion, and references cited (Supplemental Material A, see lab manual for lab report content and format). Early in the laboratory core sequence, we discuss the style of scientific writing by comparing popular press and research articles, paying particular attention to the format and content of each section of a research article. The C-Fern lab report acts as an assessment tool of this knowledge, because the students are expected to construct their lab reports in a manner similar to that of a research article and to present their data in context with the current knowledge of the field as determined from the primary literature.
ASSESSMENT
Experimental Design: Educational Goals and Student Outcomes
We developed this lab exercise to address a subset of the larger goals of the biology major core course sequence at Bucknell University. We followed the curriculum, instruction, assessment model in developing appropriate instruction and assessment techniques to meet these goals (National Institute for Science Education, 2007). The specific goals addressed by this lab exercise are that the students 1) understand the defining characteristics of the plant life cycle and plant development; 2) gain a working knowledge of experimental design; and 3) learn more effectively through the use of inquiry-based lab modules to reinforce concepts introduced in lecture, regardless of previous biology experience. We chose to focus on the plant life cycle because it is a topic that typically presents serious pedagogical challenges and a topic that can be readily approached through an inquiry-based, interdisciplinary module by using C-Fern as a model system. We designed specific measurable student outcomes based on the goals, both objective and subjective. Specifically, we expected that this lab exercise would meet the goals if, upon completion of the lab, students retained or increased their ability to identify 1) the specific stages and developmental processes that define the plant life cycle; and 2) the essential components of an experiment, including the independent and dependent variables and positive and negative controls. In addition to these objective factual measurement this lab exercise in helping them learn the required material and measured differences in attitudes between students who had taken intensive (advanced placement [AP]) biology courses, or covered similar content in high school, and those students who lacked these experiences.
Assessment Measures
Quantitative assessment of the specified outcomes was measured using a multiple-choice pretest/posttest, an attitude survey using a Likert scale, and a questionnaire regarding previous course work. The objective pretest and posttest consisted of five multiple-choice questions designed to assess student knowledge of the plant life cycle and experimental design; the questions were the same for both tests (Supplemental Material C). The tests were administered at the beginning of the lab period, before the introductory lecture, to 143 students in 10 lab sections immediately before starting the inquiry-based lab and 1 wk subsequent to its completion, a 2-wk interval. Both tests were given without prior announcement, and students were told that their performance would not affect their grade. Student names, but no other personal data, were collected. Names were used as identifiers to measure changes in student performance in the objective multiple-choice questions over the 2-wk period through analysis in a paired t test. Once this analysis was completed, the names were removed from the data to protect individual privacy. The subjective Likert-scale questionnaire was administered with the posttest (Supplemental Material C). To check the validity of this measure, that students were indeed engaging in a sincere manner with the questionnaire (Sundberg, 2002), we formulated two questions that were worded negative to each other (compare questions 11 and 12 of the posttest in Supplemental Material C). The scores of the questionnaires are reported here as median and mode; because the Likert scale is generally considered to be ordinal the mean is not an accurate measure. This protocol was approved by the Bucknell University Institutional Review Board and ruled exempt (IRB 106-146).
Assessment Results
Our assessment data indicate that this laboratory exercise helped students, both those with and those without previous exposure to similar content, to obtain the specified outcomes. The self-reported data regarding previous biological course experience show that 83% of students had covered experimental design in a previous course in college or high school. This compares to 51 and 57%, respectively, who had previous experience with the plant life cycle or who had taken AP biology. Students increased or retained their knowledge of experimental design and the plant life cycle as measured by objective multiple-choice questions (Table 3). A paired comparison of multiple-choice pretest and posttest showed that upon completion of the lab the students as a whole demonstrated a significant increase of 5.6% in their ability to correctly answer questions related to experimental design (P = 0.027); students without previous exposure to experimental design increased their score by 17.5% (P = 0.017). Students as a whole demonstrated a 3.9% (P = 0.069, not significant) increase in their ability to correctly answer the questions related to the plant life cycle and those without previous exposure to this content significantly increased their score by 6.4% (P = 0.047). It is important to note that there was no significant difference in the posttest scores between students based on their previous content exposure, suggesting that the lab helped all of the students reach an equal level of proficiency.
| Topic | Avg. score (%) | |
|---|---|---|
| Pretest | Posttest | |
| Experimental design: all students (n = 143) | 74.5 | 80.1 |
| Experimental design: no prior exposure (n = 25) | 60.0 | 77.5 |
| Plant life cycle: all students (n = 143) | 70.0 | 73.9 |
| Plant life cycle: no prior exposure (n = 73) | 67.1 | 73.5 |
The attitude questionnaire revealed that the majority of students “agreed” or “strongly agreed” that the lab improved their understanding of the subject matter, and they found that covering material in lecture and seeing it again in lab was useful (Table 4). The Likert scores for these questions were identical regardless of whether the students had taken AP biology or had previous exposure to similar content.
| Question | Likert score |
|---|---|
| This lab improved my understanding of the plant life cycle. | 4 |
| This lab improved my understanding of experimental design. | 4 |
| Covering material in lecture and seeing it again in lab is useful and helps me learn the material more effectively. | 5 |
| Covering material in lecture and seeing it again in lab is redundant and a waste of my time. | 2 |
DISCUSSION
As is true at many universities, we have gradually been replacing “cookbook” labs with inquiry-based exercises in an effort to engage students in the learning process and to increase comprehension and retention of important and challenging concepts. The lab exercise described here addresses two important goals within the curriculum of Biology 206, Organismal Biology, and of the biology core courses as a whole. The first of these goals is to provide an effective, engaging educational experience for our students within the plant sciences, specifically in regard to the unique aspects of plant development and the plant life cycle. It was our aim that such exercises will overcome the perception of students that plants are neither interesting nor relevant to their educational objectives. The second goal is to provide students with an opportunity to solve realistic scientific problems and to develop a working knowledge of the scientific method. Both goals are addressed in this lab exercise in which students propose hypotheses and design an experiment to test the specific mechanism underlying the her1 mutation of C-Fern.
C-Fern offers many benefits as a model system for undergraduate research and teaching, including ease of culture, rapid growth, and the availability of mutant strains (Renzaglia and Warne, 1995, Hickok and Warne, 2004). Furthermore, students gain experience in searching biology databases to locate primary literature that describes the her1 strain as an ACE-insensitive mutant that forms only hermaphrodites and that is unable to develop male gametophytes (Warne et al., 1988; Banks et al., 1993). C-Fern offers an obvious advantage in teaching alternation of generations because the gametophyte and sporophyte are macroscopic, free-living generations and the process of fertilization, involving the release of sperm that swim to the archegonia, can be easily observed with a compound microscope. In this lab, we do not simply use C-Fern to teach the life cycle; we also use alternation of generations as a starting point into our inquiry of the developmental processes that characterize the mutant strain. Additionally, we build on the foundation of molecular and cellular biology that students received in the previous core course.
Our assessment data indicate that this laboratory exercise helped students to obtain the specified measurable outcomes and that our instructional methodology attains the curricular goals. Students significantly improved their knowledge of experimental design, and students without previous exposure to the plant life cycle significantly improved their knowledge of this area. Students ranked this exercise as equally effective, regardless of whether they had taken AP biology in high school or whether they had covered similar content in a previous course. Students without previous exposure to these content areas obtained scores in the posttest nearly equal to those of students with previous exposure. These data suggest that this lab exercise effectively engages students with various levels of experience and helps them to equally meet the goals of the curriculum.
One of the most significant challenges in designing this lab exercise was to scale it up so that it could be carried out by 150 or more students and still provide a meaningful inquiry-based component. In the past, we offered students the opportunity to design their own “plant project” experiments using an open-inquiry approach in small groups over several weeks. However, after a few years, we discarded the plant projects in favor of the current lab exercise, because the projects were too expensive in terms of materials; they resquired too much course time and faculty time outside of the classroom; and despite our best efforts to mentor students, they often led to ill-conceived and poorly designed experiments. The lab exercise described here has elements of open inquiry in that it requires students to develop a hypothesis and to design an appropriate experiment. However, students ultimately work together as a class to carry out a fixed lab procedure with a predetermined outcome, a guided- inquiry exercise (Domin, 1999). Inquiry-based lab exercises have successfully been incorporated into the college biology curriculum (Luckie et al., 2004, Howard and Miskowski, 2005). Assessment of these lab exercises over several semesters shows that the students improved their research skills, they worked at higher cognitive levels, they performed better on standardized exams, and they reported positive attitudes regarding their learning experience. Although these studies focused on open inquiry, they relied on a structured framework that incorporated expository exercises, guided inquiry exercises, or both. We, too, have found that it is important to provide an organized framework for students to learn the scientific process and formal scientific communication. This lab exercise and other similar inquiry-based exercises in our introductory courses provide the skills that students need to engage in independent projects in upper-level courses and the research lab as supported by anecdotal evidence from our colleagues. Although this lab was designed for a large lab course for biology majors in the first year of college, it could be adapted for use in advanced college courses.
ACKNOWLEDGMENTS
We thank Drs. Sandra Field, DeeAnn Reeder, and Tristan Stayton (all at Bucknell University) for assistance in administering the pretest and posttest assessments and for valuable discussions in developing this laboratory exercise.