Getting to Evo-Devo: Concepts and Challenges for Students Learning Evolutionary Developmental Biology
Abstract
To examine how well biology majors have achieved the necessary foundation in evolution, numerous studies have examined how students learn natural selection. However, no studies to date have examined how students learn developmental aspects of evolution (evo-devo). Although evo-devo plays an increasing role in undergraduate biology curricula, we find that instruction often addresses development cursorily, with most of the treatment embedded within instruction on evolution. Based on results of surveys and interviews with students, we suggest that teaching core concepts (CCs) within a framework that integrates supporting concepts (SCs) from both evolutionary and developmental biology can improve evo-devo instruction. We articulate CCs, SCs, and foundational concepts (FCs) that provide an integrative framework to help students master evo-devo concepts and to help educators address specific conceptual difficulties their students have with evo-devo. We then identify the difficulties that undergraduates have with these concepts. Most of these difficulties are of two types: those that are ubiquitous among students in all areas of biology and those that stem from an inadequate understanding of FCs from developmental, cell, and molecular biology.
INTRODUCTION
Developmental aspects of evolution (evo-devo) form an essential part of our understanding of evolution. Some of these concepts complement the modern synthesis—for example, the concept that many types of phenotypic variation are derived from the effects of genetic variation on development (Carroll et al., 2001; Carroll, 2005; Arthur, 2011). Other concepts substantially expand our understanding of evolutionary processes, such as the concept that development can bias evolutionary outcomes and the concept that phenotypic novelty can arise via the redeployment of an existing developmental process to a novel developmental context. Recognition of the importance of evo-devo, as well as the pedagogical gains that can be made by taking an evo-devo approach in the classroom (Gilbert, 2003; Love, 2012), has fueled recent attempts to incorporate evo-devo into evolutionary biology curricula, typically as discrete, supplementary modules (Platt, 2009; but see Arthur, 2011). Evo-devo concepts, especially the conservation of HOX genes and regulatory networks across phyla, now appear in the evolution sections of high school textbooks (e.g., Miller and Levine, 2008), introductory biology courses (Sadava et al., 2010), and websites that archive teaching materials (Platt, 2009; Teachers’ Domain, 2012; Understanding Evolution, 2012a,b,c,d,e).
Evo-devo content presents students with new conceptual challenges and potential difficulties in attempting to understand evolution. For example, while several evo-devo concepts rely on the supporting concept (SC) of conserved gene networks that operate in a variety of developmental contexts, many students hold that each trait of an observed phenotype is the result of the expression of a single gene (Lewis and Kattmann, 2004). Improving strategies for teaching evo-devo will benefit from an inventory of concepts appropriate for undergraduates, a learning progression toward their mastery, and a description of their attendant conceptual difficulties.
How information is presented to a student can affect how a student reasons and assembles links between concepts (Gelman, 2003; Novak, 2006). Misconceptions arise when students inaccurately link concepts; misunderstandings arise when there are missing connections between related concepts (Ausubel, 1968; Novak, 2006). Here we use the more inclusive term conceptual difficulty to describe any conception that differs from a conception commonly held by the scientific community (Hammer, 1996a,b), including misconceptions, misunderstandings, and alternative conceptions (Wandersee and Reuter, 2006).
There is a well-established literature on the conceptual difficulties students encounter in some biological disciplines (Brumby, 1981, 1982), including genetics (Smith et al., 2008; Smith and Knight, 2012), physiology (Zuckerman, 1994a,b, 1995), and evolution. Within the latter, studies report conceptual difficulties associated with the topics of natural selection (Brumby, 1979, 1984; Bishop and Anderson, 1990; Settlage, 1994; Ferrari and Chi, 1998; Nehm and Schonfeld, 2007; Nehm and Reilly, 2007; Abraham et al., 2009; Gregory, 2009), genetic drift (Andrews et al., 2012), macroevolution (Catley and Novick, 2009), and tree-thinking (Baum et al., 2005; Meir et al., 2007; Catley et al., 2010; Morabito et al., 2010; Abraham et al., 2012). To our knowledge, there are no published inventories of the concepts necessary for undergraduate students to have a working knowledge of evo-devo, nor are there any published reports on the conceptual difficulties that students of evo-devo are likely to encounter. Our study was motivated by two questions: 1) What concepts do undergraduate students need to have a working knowledge of evo-devo? and 2) What difficulties are students likely to encounter when they attempt to learn these concepts? In this paper, we articulate core concepts (CCs), SCs, and foundational concepts (FCs) associated with evo-devo that undergraduate biology majors ought to master. We then report on conceptual difficulties that currently prevail among undergraduate students attempting to learn evo-devo. Not only will this inventory of concepts and associated conceptual difficulties help us meet the long-term goal of developing an instrument to quantify student understanding of evo-devo (e.g., Adams and Wieman, 2010), it should also help biology instructors design curricula that focus attention on core evo-devo concepts and the prerequisite concepts that enable students to avoid common conceptual difficulties.
METHODS
Identifying Evo-Devo Concepts
To identify evo-devo concepts, we began by brainstorming during a meeting of the EvoCI Toolkit Working Group at the National Evolutionary Synthesis Center (NESCent). We supplemented this initial list by reviewing the evo-devo and educational literature, drawing heavily from Hiatt et al. (2010), who surveyed biology instructors to identify the evo-devo concepts considered most important for biology majors to understand. Next, we administered a survey that asked experts to evaluate this initial list of evo-devo concepts. The survey was administered using Qualtrics (Qualtrics Labs, Provo, UT) to a group of evo-devo content experts solicited through the Evo-Devo ListServ. We defined an expert as someone who actively contributes, teaches, or conducts research in an area related to evo-devo. Thirty-six experts from a variety of institutions completed the survey, including faculty (n = 26), graduate students (n = 4), postdoctoral researchers (n = 2), and others who did not assign themselves to a category (n = 4). Experts reviewed each of the concepts by evaluating its importance for biology majors and indicating whether they teach the concept in their courses. Experts also had the opportunity to provide additional comments on each concept and describe concepts not included in the list. We used these data to revise the initial list of concepts and then compile a final, master list.
Identifying Students’ Conceptual Difficulties with Learning Evo-Devo
The conceptual difficulties that undergraduate biology majors have were compiled from several rounds of surveys and interviews. These were conducted at a variety of institutions to include a wide range of student backgrounds and regional diversity within the United States. These institutions included a private university in the Northeast (PU), a public master's comprehensive university in the Midwest (MCU), a public research-intensive university in the mid-South (RIU), and students from a private high school in the Pacific Northwest (PHS). All surveys and interviews were performed with informed consent and were deemed exempt by institutional review boards (RIU IRB nos. AS-112 and AS-125; MCU and PU also underwent IRB review, but no IRB numbers were assigned). Very few students in this survey had taken a course in developmental biology, although some upper-level students had taken a course in cell biology. A few (16%) students taking the second exploratory survey at RIU indicated they had taken a course in developmental biology or embryology, likely in the form of human reproduction or livestock reproduction, as the more general course in developmental biology had not been offered in the 4 yr preceding interviews. None of the MCU students in our sample had taken such courses. Our sample included students taking an evolution course while we collected data at MCU and RIU, comprising 11 and 31% of our total sample. In general, at all the institutions sampled, students were much more likely to be exposed to evolutionary, as opposed to developmental, biology content.
Two exploratory open-response surveys were developed (Supplemental Material, surveys 1 and 2) to elicit responses from primarily lower-level students to sets of questions addressing our list of CCs, SCs, and FCs. Each survey consisted of a description of a scenario followed by several questions. The surveys were administered to students (n = 478 students) at the institutions mentioned above either on paper or online using the local course-management software (Desire2Learn [Kitchener, Ontario, Canada], Qualtrics [Qualtrics Labs, Provo, UT], or Ed's Tools [Klymkowsky and Garvin-Doxas, 2008]). Although there was some overlap in survey items, having two distinct shorter surveys allowed us to assess a large number of students while not burdening any one student with an overly long survey. Survey 1 was administered to: 311 students from lower-level courses on animal biology, plant biology, and introductory biology and upper-level courses on evolution, stream invertebrates, anatomy and physiology, environmental toxicology, and science education research methods (MCU); and four high school seniors enrolled in a primate biology course (PHS). Survey 2 was administered to 42 students in a senior-level evolution course (RIU).
Although surveys 1 and 2 were largely administered to lower-level students, some upper-level students were included in the sample. On these initial, exploratory surveys, 96 and 86% of the responses authored by lower- and upper-level students, respectively, were incorrect and uncodable. We categorized certain incorrect responses as “uncodable” if they were incorrect as a result of being incomplete, vague, or tautological, providing insufficient context for us to determine any conceptual difficulty a student might have had (see Student Excerpts 1 for examples). This is in contrast to responses that were incorrect, because they contained one or more identifiable conceptual difficulties. This large number of uncodable responses is expected when questions are difficult to interpret or the respondent has little working knowledge needed to answer a question (Tamir, 1989). Given that many of these students have not been exposed to evo-devo concepts, it is not surprising many were unable to answer these questions sufficiently. In response to the large number of uncodable responses from the first two surveys, we revised questions for survey 3 to reduce jargon and avoid eliciting common uncodable responses (Duncan, 1979). Based on feedback from experts, questions were also revised to more precisely target concepts. Survey 3 was taken by upper-level students, and the result was a much smaller percentage (62%) of uncodable responses. Survey 3 was administered at the following institutions: MCU: 61 students in an upper-level evolution and genetics course; RIU: 39 students in upper-level evolution and vertebrate morphology courses; PU: 11 students from upper-level courses in genetics and evolution and the first course in an introductory biology sequence.
Data from all three exploratory surveys were analyzed for discernible patterns in student responses (Berelson, 1952). We determined whether students consistently gave similar answers for each question and also identified conceptual difficulties that consistently prevented students from answering a question correctly. Finally, we identified instances in which we were unsure of the source of error in a student's response and pursued these with student interviews. Surveys 1 and 2 were mostly administered to lower-level students and did not contain questions addressing all the CCs that appear in survey 3, which was administered primarily to upper-level students. Therefore, to calculate the frequency of conceptual difficulties, we only used student responses from survey 3. To confirm the understandings or conceptual difficulties we inferred based on written responses, we constructed survey 4 (interview only) to address more closely some of the concepts with which students struggled (Supplemental Material, survey 4). For example, in cases in which students tended to rely exclusively on natural selection in their written responses, we wanted to determine whether natural selection was merely the students’ first inclination or actually represented the full extent of their knowledge. Think-aloud interviews were conducted (Patton, 2002) to give students the opportunity to define and explain their terminology while providing information about how they formulated explanations. To prevent participant fatigue, we broke the questions down into subsets so that interviews would last no more than 30 min. Survey 4 was administered to upper-level students at RIU who identified as biology majors and included one zoology graduate student. Research assistants transcribed the audio recordings from those interviews. Data were analyzed using NVivo 9 (QSR International, Cambridge, MA).
Following interviews, we used emergent coding (Haney et al., 1998) to organize the conceptual difficulties into four categories: common biological (CB), developmental (DV), evolutionary (EV), and evo-devo (ED). It is important to note that we used the term “developmental” (DV) as shorthand to include related conceptual difficulties in cell and molecular biology as well. The process of categorizing conceptual difficulties was necessarily iterative to ensure agreement among investigators. To begin, two of us (A.H. and K.E.P.) independently assigned the same subset of responses (19.02%) to one or more categories of conceptual difficulties. We compared our assignments, and for any category of conceptual difficulty that had >25% disagreement, we revised our definition of the category and re-evaluated the student responses to determine whether they belonged in the revised category. This revise-and-discuss process was continued until there was >95% agreement between the two investigators for the entire subset of responses, suggesting minimal bias (Stemler, 2001; Patton, 2002). After agreement was reached, one investigator (A.H.) coded the remaining data.
RESULTS
We used the literature and data from the survey of experts to identify evo-devo concepts that could frame future evo-devo teaching. We then examined open responses to survey questions and conducted interviews to identify conceptual difficulties that students experienced when trying to learn these concepts. Some of the frequently encountered conceptual difficulties were common to several subdisciplines of biology, whereas others stemmed from an inadequate understanding of development.
Evo-Devo Concepts
Despite the variety of topics that fall under the umbrella of evo-devo, we found a broad consensus among experts on which evo-devo concepts undergraduate biology majors ought to know: 92.6% of our initial evo-devo concepts were deemed either “critical” or “important.”
The survey data helped us delineate the hierarchical categories of CC, SC, and FC. Each CC relies on one or more SCs, which are divided into subcategories that rely on one or more FCs from developmental, cell, and molecular biology on the one hand, or the modern synthesis on the other (Figure 1). All told, we identified six core, 19 supporting, and six foundational evo-devo concepts.
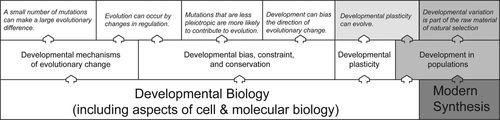
Figure 1. Schematic depicting how CCs in evo-devo (upper layer) rely on different subcategories of SCs (middle layer), which in turn rely on FCs from both developmental biology, including cell and molecular biology, and evolutionary biology/the modern synthesis (lower layer). Arrows indicate specific dependencies between the CCs and types of SCs and FCs.
Question: You found a chicken egg, you hatched it out and observed that the chicken has a beak. Can you describe how the chicken's beak was formed? [Note: This is the first in a series of questions in which the questions become increasingly more specific in an attempt to elicit developmental knowledge. See Supplemental Material for the complete series.]
Example of a student response exhibiting developmental understanding:
Student: The chicken beak was formed through a large amount of cell differentiation in development. Genes expressed by the chicken caused cells to differentiate into a beak.
Examples of student responses that were considered uncodable follow. This category included responses that were deemed insufficient because the response was vague and did not provide enough information to identify any specific conceptual difficulty.
Student 1: It was developed in the embryo.
Student 2: DNA→Protein→Beak
Student 3: No, I am not sure how to describe how a beak is formed.
Student 4: The chicken's beak was formed during the development of the chick inside the egg.
Student 5: Genetics.
Student 6: During gestation.
Student 7: Embryonic tissue.
Although most of the concepts we identified were deemed essential for understanding evo-devo, the list of concepts is not exhaustive. To explore student conceptual difficulties, we found it necessary to limit the number of concepts examined. Therefore, we elected not to examine FCs from evolutionary biology that have been articulated elsewhere (e.g., Khodor et al., 2004). We also elected not to include concepts that were not consistently agreed upon in the expert survey as being essential for undergraduates attempting to gain a basic understanding of evo-devo, even though some are arguably of great importance evolutionarily. These included canalization (Waddington, 1959); genetic assimilation and accommodation (Schmalhausen, 1949; Waddington, 1959; West-Eberhard, 2003; although see CC6); epigenetic modification of DNA; gene duplication and genome evolution (Lynch, 2007); serial homology; modularity (Schlosser and Wagner, 2004); facilitated evolution (Gerhart and Kirschner, 2007); and the evolution of multicellularity (Grosberg and Strathmann, 2007).
Each of the six CCs we examined is made explicit in Table 1. Collectively, they are integrative concepts in evo-devo and range from simpler concepts, such as the mere inclusion of development into the process of natural selection on variation (CC6), to more complex concepts, such as the notion that changes in the regulation of developmental processes can be a source of evolutionary novelty (CC2), that such changes can sometimes result from a small number of mutations (CC1), or that development can bias the direction of evolutionary change (CC4).
| CCs in evo-devo | Conceptual difficultiesa |
|---|---|
| CC1. A small number of mutations can make a large evolutionary difference: It is possible for novel phenotypes to evolve as the result of the fixation of a small number of mutations that cause significant changes in the regulation of developmental processes.b This does not preclude the possibility that many (or even most) differences between species require a large number of small effect mutations. | CB1, CB2, CB5, DV1*, EV1, EV4, EV5, EV7, ED1, ED2, ED3 |
| CC2. Evolution can occur by changes in regulation: Given that developmental processesb are often shared, novel phenotypesc often evolve via changes in regulation (e.g., co-option or deployment of gene regulatory networks to different tissues or stages of development). | CB1**, CB2, CB4, CB5, DV1*, DV2, DV5, EV2, EV4, EV5, EV6, EV8, ED1, ED2, ED3 |
| CC3. Mutations that are less pleiotropic are more likely to contribute to evolution: Mutations that are less pleiotropic (e.g., mutations in a gene or gene product that plays only a limited role in development, in a modular cis-regulatory element, or in a modular domain of a protein) are less likely to have deleterious pleiotropic effects on fitness and thus are more likely to become fixed in populations. | CB1**, CB2, DV1*, DV2, DV4, EV2, EV4, EV11, ED1, ED2, ED3, ED4 |
| CC4. Development can bias the direction of evolutionary change: Developmental processesb can bias evolutionary outcomes by either limiting the variation available to natural selection or attaching deleterious pleiotropic effects to certain variants. | CB1, CB2, CB4, CB5, CB6, DV1*, DV2, DV3, DV4, EV1**, EV2, EV3, EV4, EV5, EV6, EV8, EV9, EV10, ED1, ED2, ED3, ED4 |
| CC5. Developmental plasticity can evolve: The environment can select among heritable variation in a developmental response to a particular environmental change, resulting in adaptive developmental plasticity. | CB2*, CB4, DV1**, DV2, DV3, EV2, ED1 |
| CC6. Developmental variation is part of the raw material of natural selection: Many adaptations are the result of the environment selecting among heritable variation in phenotypec that is the result of heritable variation in developmental processes,b which is itself the result of genetic variation. | CB1, CB2, CB4, CB5, DV1*, DV2, DV4, DV5, EV2, EV4, EV11, ED1, ED2, ED3, ED4 |
These CCs rely on SCs from subcategories that are divided further in Table 2: developmental mechanisms of evolutionary change (SCa); developmental bias, constraint, and conservation (SCb); developmental plasticity (SCc); and development in populations (SCd). These SCs in turn rely on FCs in development and evolution. Each of the SCs and FCs and their subcategories is made explicit in Table 2.
| SCs essential for understanding evo-devo | Conceptual difficultiesa |
|---|---|
| Developmental mechanisms of evolutionary change (subcategory a) | |
| SCa1. A change in the role a gene plays in a developmental processb can lead to a change in phenotypec (by changing the nature, timing, or place of the developmental process). | CB1**, CB2, CB4, CB5, CB6, DV1*, DV2, DV3, DV4, EV1, EV2, EV3, EV4, EV5, EV7, EV8, EV9, EV10, EV11, ED1, ED2, ED3, ED4 |
| SCa2. The role a gene plays in a developmental processb can change due to the fixation of DNA mutation(s) that alter either: 1) the regulation of the gene,d 2) the regulation of a gene's product,e,f or 3. the sequence of the gene's product. | CB1**, CB2, CB3, CB4, CB5, CB6, DV1*, DV2, DV3, DV4, DV5, EV1, EV2, EV4, EV5, EV6, EV7, EV8, EV9, EV11, ED1, ED2, ED3 |
| SCa3. Significant changes in regulation (either gene regulation or regulation of the gene product) allow for homologous genes and gene products to have multiple and distinct roles in different species. | CB1**, CB2, CB4, CB5, DV1*, EV1, EV2, EV3, EV4, EV5, EV7, EV8, EV10, ED1, ED2, ED3, ED4 |
| SCa4. Significant changes in regulation (either gene regulation or regulation of the gene product) can result from the fixation of a small number of mutations. | CB1**, CB2, CB3, CB4, CB5, DV1*, DV2, DV5, EV1, EV2, EV3, EV4, EV5, EV6, EV7, EV8, EV10, EV11, ED1, ED2, ED3, ED4 |
| Developmental bias, constraint and conservation (subcategory b) | |
| SCb1. Homologues of genes are often present in the genomes of distantly related species. | CB1**, CB2, CB3, CB4, CB5, CB6, DV1*, DV2, DV3, DV4, DV5, EV1, EV2, EV3, EV4, EV5, EV6, EV7, EV8, EV9, EV10, EV11, ED1, ED2, ED3, ED4 |
| SCb2. Homologous developmental processesb (involving homologous genes) can occur during the development of different, often distantly related, species and constitute a shared developmental “toolkit.” | CB1**, CB2, CB3, CB4, CB5, DV1*, DV2, DV3, DV4, DV5, EV1, EV2, EV3, EV4, EV5, EV6, EV7, EV8, EV9, EV10, EV11, ED1, ED2, ED3, ED4 |
| SCb3. The possession of similar developmental processesb by different species can result from shared ancestry and selection to maintain those processes. | CB1, CB2, CB4, CB5, CB6, DV1*, DV2, DV3, DV4, EV1**, EV2, EV3, EV4, EV5, EV6, EV8, EV9, EV10, EV11, ED1, ED2, ED3, ED4 |
| SCb4. Due to the integration and interdependence of developmental processes,b certain character variants will not contribute to variation in a population if they are developmentally impossible or inviable. | CB1, CB2, CB4, CB5, CB6, DV1*, DV2, DV3, DV4, EV1**, EV2, EV3, EV4, EV5, EV6, EV8, EV9, EV10, EV11, ED1, ED2, ED3, ED4 |
| SCb5. Due to the integration and interdependence of developmental processes,b certain character variants are viable but suffer lower fitness due to deleterious pleiotropic effects, regardless of the environment. | CB1, CB2, CB4, CB5, DV1*, EV1**, EV2, EV3, EV4, EV6, EV8, EV10, ED1, ED2, ED4 |
| SCb6. The integrated and interdependent nature of development can result in the general conservation of some phenotypes,c including body plans. | CB1**, CB2, CB3, CB4, CB5, CB6, DV1*, DV2, DV3, DV4, DV5, EV1, EV2, EV3, EV4, EV5, EV6, EV7, EV8, EV9, EV10, EV11, ED1, ED2, ED3, ED4 |
| Developmental plasticity (subcategory c) | |
| SCc1. Changes in the environment can induce a change in development that results in a change in phenotype. | CB1, CB2**, CB4, CB6, DV1*, DV2, DV3, DV4, EV2, EV4, EV7, EV8, EV9, ED1 |
| SCc2. The magnitude and nature of developmental responses to the environment can vary among individuals in a population. | CB1, CB2**, CB4, CB6, DV1*, DV2, DV3, DV4, EV2, EV4, EV7, EV8, EV9, ED1 |
| SCc3. Variation in developmental response to the environment can be due to mutation(s) in DNA and can thus be heritable. | CB1, CB2**, CB4, CB6, DV1*, DV2, DV3, DV4, EV2, EV4, EV7, EV8, EV9, ED1 |
| Development in populations (subcategory d) | |
| SCd1. The nature, timing, or location of a developmental processb can vary among individuals in a population. | CB1**, CB2, CB3, CB4, CB5, DV1*, DV2, DV5, EV1, EV2, EV4, EV5, EV6, EV7, EV8, EV11, ED1, ED2, ED3 |
| SCd2. A developmental processb can vary due to variation in the roles of the genes and gene products that participate in that process. | CB1**, CB2, CB5, DV1*, EV1, EV4, EV5, EV7, ED1, ED2, ED3 |
| SCd3. The role a gene plays in a developmental processb can vary within a population due to variation in the regulation of the gene,d in the regulation of the gene's product,e,f or the sequence of the gene's product. | CB1, CB2, CB4, CB5, DV1*, DV2, DV4, EV1, EV2, EV3**, EV4, EV8, EV10, EV11, ED1, ED2, ED3, ED4 |
| SCd4. If due to mutation(s) in DNA, variation in the regulation of a gene or a gene product is heritable. | CB1, CB2, CB5, DV1*, EV1, EV4, EV5, EV7, ED1, ED2, ED3 |
| SCd5. Variation in the sequence of a gene's producte is most often due to mutation(s) in DNA and is thus heritable. | CB1, CB2, CB5, DV1*, EV1, EV4, EV5, EV7, ED1, ED2, ED3 |
| SCd6. Variation in developmental processesb can be heritable. | CB1**, CB2, CB3, CB4, CB5, CB6, DV1*, DV2, DV3, DV4, DV5, EV1, EV2, EV4, EV5, EV6, EV7, EV8, EV9, EV10, EV11, ED1, ED2, ED3, ED4 |
| Developmental biology (subcategory a), including aspects of cell and molecular biology | |
| FCa1. Developmental processesb (including maternally directed processes) are the proximate causes of phenotype.c | CB1**, CB2, CB3, CB4, CB5, CB6, DV1*, DV2, DV3, DV4, DV5, EV1, EV2, EV3, EV4, EV5, EV6, EV7, EV8, EV9, EV10, EV11, ED1, ED2, ED3, ED4 |
| FCa2. During development, the expression of different sets of genes in different cells results in different cell types. | CB1**, CB2, CB3, CB4, CB5, CB6, DV1*, DV2, DV5, EV1, EV2, EV4, EV5, EV6, EV7, EV8, EV9, EV11, ED1, ED2, ED3 |
| FCa3. Developmental processesb involve complex interactions between genes and gene products (e.g., within gene regulatory networks), cells, and tissues. | CB1, CB2, CB5, DV1*, EV1, EV4, EV5, EV7, ED1, ED2, ED3 |
| FCa4. The role a gene plays in a developmental processb is determined by: 1) regulation of the gene,d 2) regulation of the gene's product,e,f and 3) interactions between the gene's product and other genes or gene products. | CB1**, CB2, CB3, CB4, CB5, CB6, DV1*, DV2, DV3, DV4, DV5, EV1, EV2, EV3, EV4, EV5, EV6, EV7, EV8, EV9, EV10, EV11, ED1, ED2, ED3, ED4 |
| FCa5. As part of a network, a gene can function in developmental processesb that take place in different cells or tissues at different stages of development (i.e., the gene can be pleiotropic). | CB1, CB2, CB4, CB5, DV1*, DV2, DV4, EV1, EV2, EV3**, EV4, EV8, EV10, EV11, ED1, ED2, ED3, ED4 |
| FCa6. A modified developmental processb often results in a modified phenotype (but not always). | CB1**, CB2, CB3, CB4, CB5, CB6, DV1*, DV2, DV3, DV4, DV5, EV1, EV2, EV3, EV4, EV5, EV6, EV7, EV8, EV9, EV10, EV11, ED1, ED2, ED3, ED4 |
Conceptual Difficulties
Several examples of student conceptual difficulties, as illustrated by excerpts of student responses from open-response surveys and interviews, are shown below. Table 3 summarizes the conceptual difficulties we identified. Figure 2 shows the percentage of correct responses, uncodable responses, and responses containing conceptual difficulties for questions targeting each of the CCs and subcategories of SCs and FCs. Uncodable responses included those responses that were incorrect because they were incomplete, vague, or tautological, with the result that they did not provide enough context to determine any conceptual difficulty that a student might have (see Student Excerpts 1 for examples).
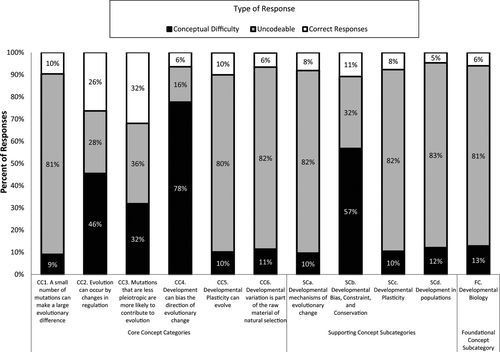
Figure 2. Frequency of different types of student responses (possessing conceptual difficulty, uncodable, and correct) to questions targeting each of the CCs, the four categories of SCs, and FCs in developmental biology. Total number of responses was 4536.
| Conceptual difficulties | Number of responses | |
|---|---|---|
| Common biological (CB) | 192 (26%) | |
| CB1. Teleology. | Attributing design and purpose to organism, environment, process, or mechanism. Responses that exhibit this difficulty include references to purpose or design. | 109 |
| CB2. Vocabulary. | Misusing terms (e.g., confusing gene, allele, and genome). | 66 |
| CB3. Anthropomorphism. | Attributing human qualities to nonhuman organisms, environments, processes, or mechanisms. | 3 |
| CB4. Negative connotation. | Attributing a negative relationship with an organism, environment, or process, e.g., “all mutations are bad” or “mutants suffer or are deformed.” | 15 |
| CB5. Essentialism. | Providing or assuming a set of properties that the organism, environment, or process must possess to qualify as a member of a category or class. | 21 |
| CB6. Personification. | Personifying a nonhuman organism, environment, mechanism, or process. | 4 |
| Developmental (DV), including cell and molecular biological aspects | 305 (41%) | |
| DV1. Lack of development.* | Failing to reference development, even when prompted. Includes invoking natural selection as a mechanism in place of more appropriate evo-devo mechanisms. | 270 |
| DV2. A single gene affects a single trait. | Stating explicitly or implying that each trait is determined by a single gene or that each gene determines only one trait. | 13 |
| DV3. Genes products are organismal phenotypes. | Stating explicitly or implying that genes are trait-bearing, that the products of genes are organismal phenotypes. No mention of transcription/translation, proteins, gene interactions, or development. | 15 |
| DV4. Environment is irrelevant to phenotype. | Stating explicitly or implying that only genes control phenotypes; plasticity/environmental influence does not influence development. | 10 |
| DV5. HOX genes are the only regulatory genes.* | Stating explicitly or implying that HOX genes are the only regulatory genes. | 4 |
| Evolutionary (EV) | 124 (17%) | |
| EV1. Characteristics that are not used are lost. | Implying that characteristics that are not used by the organism are lost simply because they are not used and not because of the loss of maintenance selection. | 33 |
| EV2. Inheritance of acquired traits. | Implying that evolution proceeds by the inheritance of acquired characteristics. Among the latter, we do not include potentially legitimate examples, such as the genetic assimilation of induced phenotypes (Waddington, 1959) or the assimilation of learned behaviors, as in the Baldwin Effect (Weber and Depew, 2003). | 30 |
| EV3. Lack of selection results in stasis.* | Stating explicitly or implying that evolutionary stasis occurs only when selection (either stabilizing or positive) does not occur. | 13 |
| EV4. Lack of understanding of population-level processes. | Statement implies a lack of understanding of population-level processes. For example, attributing evolutionary adaptation, the population-level process, to an individual. | 28 |
| EV5. All evolution results in adaptation. | Stating explicitly or implying that all evolution results in adaptations, ignoring the possibility of adaptively neutral changes. | 6 |
| EV6. Exclusive gradualism. | Stating explicitly or implying that that all changes in the phenotype must evolve gradually. | 4 |
| EV7. There is a perfect phenotype. | Stating explicitly or implying that natural selection results in a perfect phenotype. | 12 |
| EV8. Positive natural selection is the only mechanism of evolution. | Stating explicitly or implying that the only mechanism of evolutionary change is positive selection for a trait, ignoring the possibility of genetic drift. Note that this is similar to EV5 but concerns process, rather than pattern. | 10 |
| EV9. Selection acts on genes, not the phenotype. | Stating explicitly or implying that that selection acts on genes independently of the phenotype. | 6 |
| EV10. Older clades are more morphologically diverse.* | Stating explicitly or implying that older clades are always more morphologically diverse, or the converse, that younger clades are less morphologically diverse. | 14 |
| EV11. Defines selection incorrectly. | Defining selection as the ability to pass successful genes to offspring, as any trait that increases fitness (rather than the process), as a mutation that results in a “better” species, as the survival of individuals with the most adaptations, as the result of competition, or as the environment choosing the best adaptations. | 4 |
| Evo-Devo (ED) | 56 (8%) | |
| ED1. Changes in gene expression result only from mutations in said gene.* | Stating explicitly or implying that a change in a gene's expression must be due to a mutation in the cis-regulatory enhancers of that gene; not recognizing the potential for mutations in upstream regulators (in trans) to alter expression. | 20 |
| ED2. Gene expression evolves only when genes appear or disappear.* | Stating explicitly or implying that gene expression evolves only because a gene appears or disappears. | 14 |
| ED3. Phenotypic change can only result from a gene appearing or disappearing.* | Stating explicitly or implying that phenotypic change only occurs when genes appear or disappear in the genome. | 13 |
| ED4. Only closely related species have conserved traits.* | Stating explicitly or implying that only closely related species can have conserved genes, proteins, or developmental processes. | 5 |
For questions targeting each of these CCs and subcategories, students responded correctly less than 35% of the time (Figure 2). Correct responses were most common for questions targeting CC2 (26%) and CC3 (32%), while questions targeting all other concepts elicited a correct response rate equal to or less than 11%, with the lowest correct response rates for questions targeting CC4 (6%) and CC6 (6%).
Question: Insects such as the fruit fly Drosophila possess three pairs of legs, while other arthropods (e.g., Artemia, brine shrimp) can possess many more pairs of legs. In all arthropods, including insects, Dll is a regulatory gene that is required for leg formation. Provide an explanation for why Drosophila has fewer legs than Artemia.
Student response exhibiting evo-devo thinking:
Student: Dll is not as active in Drosophila, but is upregulated (or more active) in species like Artemia.
Student response exhibiting teleological thinking (CB1):
Student: No need for that many legs.
Student response that is correct but incomplete because it lacks developmental thinking (DV1):
Student 1: Flies have fewer legs because as years have passed, their bodies have changed in order to better fit its needs and that many legs wasn't necessary in order for the flies to survive.
Student 2: The environment, including habitat and food, of Drosophilia [sic] provides better chance of the individuals with fewer legs to survive. Thus, throughout the process of natural selection, among the various types of population due to genetic mutations, the ones with three pairs of legs survived more than the ones with more legs, and eventually weeding out the latter.
We found that questions targeting CC4, SCb, and CC2 had the highest percentages of responses displaying a conceptual difficulty (78, 57, and 46%, respectively; Figure 2). To understand the types of difficulties students expressed, we examined the prevalence, in upper-level students, of the four categories of conceptual difficulty among targeted concepts (Figure 3). The prevalence of common biological (CB) conceptual difficulties ranged between 40% for CC1 to 13% for CC3. Among specific CB conceptual difficulties, the most prevalent (15%) was the use of teleology or the implication that organisms evolve to achieve a purpose (CB1; see Student Excerpts 2 for an example). Smaller proportions of student responses indicated anthropomorphism (CB3; 0.4%) or essentialism (CB4; 2.0%).
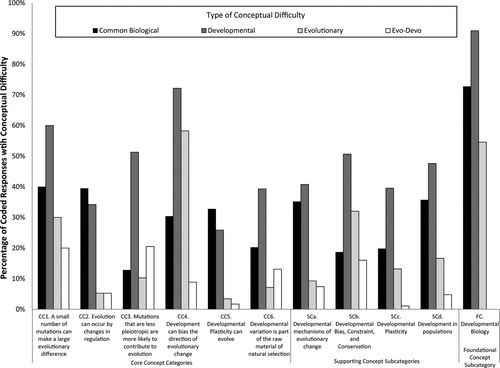
Figure 3. Prevalence of types of conceptual difficulties (i.e., percent of responses exhibiting a type of conceptual difficulty) encountered for questions targeting each of the CCs, the four categories of SCs, and FCs in developmental biology. Note that a student response may include more than one conceptual difficulty and that the figure does not include uncodable responses. Total number of codable responses was 633.
In addition to these common biological conceptual difficulties, many conceptual difficulties interfere specifically with the integration of development and evolution. For example, a student reasoning with minimal or incorrect information about the developmental mechanisms of evolutionary change (SCa) will have difficulty understanding how evolution can occur by changes in regulation (CC2). In this respect, conceptual difficulties that stem from poor or limited knowledge of development (DV) were the most prevalent overall; of 742 student responses from survey 3, 305 indicated a conceptual difficulty associated with developmental, cellular, or molecular biology (Table 3). Of these, 270 did not include any developmental reasoning, even when such reasoning was appropriate or the questions specifically prompted such reasoning (DV1, see Student Excerpts 2 and Student Excerpts 3 for examples). Instead, many of these responses (99) relied solely on natural selection as an explanatory mechanism. Even when informed during the interview that selection is an inadequate explanation, students (in interviews) still rely solely upon natural selection in their explanations (see Student Excerpts 3).
Question: All centipedes have an odd number of leg-bearing segments. Centipedes vary in the number of leg-bearing segments, from as few as 5 to as many as 125, but none possess an even number. How might you explain this fact?
A student response that exhibits a lack of developmental thinking (DV1) with exclusive reliance on natural selection and the misuse of a term from genetics (CB2) follows.
Student: I would say they all might be odd because in some way it would be advantageous to their environment for how it came about and then it stayed that way because it never became disadvantageous … The genes for an even number of segments might just be so recessive that it's basically impossible to get them.
We observed a similar pattern when we examined the prevailing conceptual difficulties among responses to questions targeting the CCs and subcategories of SCs. DV conceptual difficulties were the most prevalent for most question types, other than those targeting CC2 and CC5 (Figure 3). Consistently, responses to questions targeting the core concepts CC1, CC3, CC4, and CC6, as well as SCs belonging to the subcategories SCb and SCd, showed the greatest prevalence of DV conceptual difficulties.
Another notable challenge for students was vocabulary. In survey 3, students misused terms in 8.9% of codable responses, and most of these misused terms were from developmental biology or genetics. For example, students often used the terms “gene,” “allele,” and “genome” interchangeably in written responses and failed to distinguish among them when pressed in follow-up interviews. Students also conflate the contemporary use of the term “gene expression” (i.e., transcription) with the phenotypic expression of an allele. This conflation hampers the ability to understand how changes in phenotype can result from changes in the regulation, and potentially expression, of a gene (CC2).
DISCUSSION
The recent integration of evolution and development began with the advent of the synthetic field of evo-devo in the 1980s (Arthur, 2011). This wave of integration, however, has yet to permeate undergraduate life sciences curricula, in which traditional course structures unintentionally foster the tendency of students to compartmentalize knowledge rather than connecting it across traditional disciplines. We propose that the conceptual hierarchy presented in Figure 1 is also a pedagogical hierarchy that reflects the need for students to integrate in order to achieve a working knowledge of evo-devo. This hierarchy provides a “developmental corridor” (Brown and Campione, 1996) through which educators can guide students (e.g., Catley et al., 2004) away from the many conceptual difficulties that challenge students attempting to learn evo-devo. These include difficulties that are common across biological disciplines, as well as those that are specific to the integration of evolution and development. We discuss below the implications of these findings for effectively teaching evo-devo.
A Pedagogical Framework for Evo-Devo
Evo-devo is popularly used (Carroll, 2005) to demystify the origins of novelty (Gilbert, 2003) and explain the underlying developmental mechanisms of evolution; however, students must have the supporting and foundational conceptual framework in order to articulate evo-devo concepts correctly. For example, CC2—changes in the regulation of developmental processes can be a source of evolutionary novelty—relies on several SCs from the subcategory developmental mechanisms of evolutionary change (SCa), including the concept that a change in the role a gene plays in development can lead to a change in phenotype (SCa1), which in turn relies on several FCs from development (FCa), including the concept that a gene's role in development can be altered by, among other things, a change in the regulation of the gene (FCa4). Our results suggest that many students fail to integrate concepts from development, genetics, and evolution, and as a result, retain gaps or incorrect links in their conceptual understanding of evo-devo. This lack of integration is ubiquitous among science and humanities disciplines and is a common challenge for college educators (National Research Council, 2000).
To assist instructors in helping students make these links, we suggest that the hierarchical framework of evo-devo concepts in Figure 1 be used as a pedagogical framework. For example, before teaching the concept that less-pleiotropic mutations are more likely to contribute to evolution (CC3), one must ensure that students possess the SCs and FCs that undergird this CC—for example, deleterious pleiotropic effects (SCb4 and SCb5) and the ability of genes to play multiple roles during development (FCa5). Students who do not have a foundational understanding of the complex and interdependent roles that genes play in development may struggle to understand how development can influence the evolutionary process.
Common Biological Conceptual Difficulties: Obstacles to Evo-Devo and Then Some
Previous work has demonstrated that students struggling to learn biology, and indeed science, often resort to a set of common conceptual difficulties (Jungwirth, 1977; Bishop and Anderson, 1990; Carmichael et al., 1990; Pfundt and Duit, 1991; Tamir and Zohar, 1991; Demastes et al., 1995). These include students’ nonscientific ideas about the natural world that are based on common experiences (diSessa, 1993). Several of these have been studied in the area of evolution, including teleology, anthropomorphism, essentialism, and personification (Jungwirth, 1975; Brumby, 1979; Kargbo et al., 1980; Brumby, 1984; Clough and Wood-Robinson, 1985; Halldon, 1988; Lawson and Thompson, 1988; Bishop and Anderson, 1990; Greene, 1990; Demastes et al., 1996; Jensen and Finley, 1996; Settlage and Jensen, 1996; Samarapungavan and Wiers, 1997; Anderson et al., 2001, 2002; Southerland et al., 2001; Stewart and Rudolph, 2001; Passmore and Stewart, 2002; Sinatra et al., 2003). Our study confirms that these misconceptions also interfere with the ability of students to understand concepts in evo-devo (Table 3 and Figure 3). In particular, invoking purpose or need as a mechanism (CB1, teleology) was a common conceptual difficulty among our student responses, with 14.7% students unable to provide any mechanism other than “it was needed” (see Student Excerpts 2). Although these notions are not specific to evo-devo, instructors in this area should be aware of how such notions shape the way students understand the world.
Foundational Conceptual Difficulties: Obstacles to Integration
Because our conceptual framework is hierarchical (Figure 1), any conceptual difficulty associated with a SC or FC may propagate. In particular, we found that students often have difficulty understanding core and supporting evo-devo concepts, because they have conceptual difficulties with FCs from development. Two types of evidence from our surveys support this claim: 1) the concepts targeted by the questions that elicited the lowest correct response rates (Figure 2) and 2) the relative prevalence of different types of conceptual difficulty (Figure 3).
One of the lowest correct response rates we observed was for questions targeting the CC that development can bias the direction of evolutionary change (CC4), for which only 6% of responses were deemed correct (Figure 2). For CC4, this low rate was likely due to the fact that these questions, more than any others, required that students invoke some sort of developmental constraint as opposed to relying solely on natural selection (see Student Excerpts 2 and Student Excerpts 3 for examples of such questions). Questions targeting SCs belonging to the subcategory developmental bias, constraint, and conservation (SCb) did not receive comparably low correct response rates, because they targeted the SCs that genes and developmental processes are often shared (e.g., SCb1–2), without targeting the evolutionary consequences of these concepts.
An equally low correct response rate (6%) was for questions targeting the CC that developmental variation is part of the raw material of natural selection (CC6; Figure 2). Although many of the students we surveyed were able to describe accurately the process of natural selection and the inheritance of genetic material, they often faltered when asked to describe the process by which mutations can alter phenotype and how variation in this process can be selected over generational time. The lack of understanding of this category of knowledge is probably partially explained by the low (5–6%) correct response rates for questions targeting SCs in the subcategory development in populations (SCd), as well as FCs in development. Without this supporting and foundational knowledge, core evo-devo concepts are out of reach.
The second type of evidence supporting our claim that difficulties with FCs in developmental biology are the primary obstacles for students learning evo-devo is the relative prevalence of different types of conceptual difficulty. For all question types, save those targeting CC2 and CC5, developmental (DV) conceptual difficulties were the most prevalent (Figure 3). In particular, this was true for the question types that elicited the most conceptual difficulties overall—namely, those targeting the CCs that a small number of mutations can make a large evolutionary difference (CC1) and that development can bias the direction of evolutionary change (CC4), as well as SCs in the subcategory “developmental bias, constraint, and conservation” (SCb). Not surprisingly, the highest percentage of DV conceptual difficulties were elicited by questions targeting foundational developmental biology concepts (FC). Again, the high prevalence of conceptual difficulties—in particular DV conceptual difficulties—among these responses is due, in part, to the fact that these questions tended to require that students reference development in their answers.
A specific example of the challenge presented by poor or limited knowledge of development is the persistent misconception that “a single gene affects a single trait”; that is, that each trait has a single gene responsible for its development or that each gene is responsible for the development of a single trait (DV2 in Table 3; 1.8% of responses). This conceptual difficulty effectively precludes students from understanding any CCs or SCs that rely on the notion of development as a complex, interdependent process (e.g., CC2, SCb4, SCb6, SCd2, and SCd3) or the notion of pleiotropy (e.g., CC3 and SCb5). The most prevalent specific conceptual difficulty among student responses, however, was “lack of development” (DV1 in Table 3). Although this conceptual difficulty was inferred only when questions prompting developmental answers elicited responses that made no reference to development at all, this single problem still accounted for almost half of all instances of conceptual difficulty among all responses.
In addition to conceptual difficulties with development, we also detected conceptual difficulties with evolution, though these were not as prevalent (Figure 3). Several other studies have shown that students often struggle to fully understand evolutionary concepts (Brumby, 1979, 1984; Bishop and Anderson, 1990; Settlage, 1994; Ferrari and Chi, 1998; Baum et al., 2005; Meir et al., 2007; Nehm and Schonfeld, 2007; Nehm and Reilly, 2007; Abraham et al., 2009, 2012; Catley and Novick, 2009; Gregory, 2009; Morabito et al., 2010; Andrews et al., 2012), and it is perhaps surprising that we did not detect at least an equal prevalence of evolutionary conceptual difficulties. A real possibility is that this disparity reflects how undergraduates typically learn evo-devo—namely, as a discrete module in the context of a course on evolution, wherein evolutionary conceptual difficulties are more likely to be confronted, but developmental conceptual difficulties are allowed to persist.
Natural Selection as a Fallback Strategy
An interesting consequence of a poor or limited knowledge of developmental biology is the tendency of students to invoke natural selection as a fallback strategy, even when such an answer is inappropriate. This strategy appeared in responses to questions that specifically called for proximate mechanisms to explain phenotypes, such as those targeting the SCs in the subcategory development in populations (SCd), as well as FCs from development (FCa). This fallback strategy also appeared in responses to question scenarios that actually precluded natural selection as the sole explanatory mechanism. Students who lacked access to developmental mechanisms typically responded to scenarios of evolutionary stasis that called for a version of developmental constraint (e.g., questions targeting CC4 or SCa) by invoking a historical absence of selection (see Student Excerpts 3 for an example).
Curricular Implications
To address students’ lack of molecular and developmental knowledge necessary to formulate successful evo-devo explanations for evolutionary phenomena, we propose college-level curricula emphasize such concepts in lower-level and introductory biology courses. For example, when introducing students to DNA transcription, instructors could additionally cover the mechanisms of gene regulation. Courses that survey organismal diversity typically cover basic evolution. With minor additions of content, the evolutionary discussion in such courses could move beyond population-level mechanisms to introduce students to the idea of evolution by changes in regulation, using a relatively straightforward example, such as the roles of the genes Ultrabithorax and Distalless in the evolutionary loss of abdominal appendages in insects (Ronshaugen et al., 2002). We found that students are unlikely to graduate with sufficient understanding of evo-devo if explicit instruction about evo-devo concepts is restricted to distinct courses on evolution or development rather than being taught throughout life sciences curricula. The best way to integrate evo-devo across biology curricula, however, has yet to be determined.
In addition to suggesting a need for quality evo-devo instruction, our data also indicate a gap in student ability in science practice. Our data suggest students are not making connections across the content areas of development and evolution. The ability to tap into the interdisciplinary nature of science is one of the core competencies proposed by the Vision and Change report (American Association for the Advancement of Science, 2009) as necessary for science students. Efforts to improve student ability to integrate information could target evo-devo foundational, supplementary, and CCs, as these are ideas that students fail to integrate. By using the provided framework of concepts and attendant conceptual difficulties, instructors can implement student-centered approaches in the classroom to diminish barriers to interdisciplinary thinking and achieve a better conceptual understanding of evo-devo.
CONCLUSION
This is the first study to propose a framework for building a working knowledge of evo-devo in undergraduate biology education. The framework is a hypothesis that requires further testing to determine whether or not the proposed dependencies between CCs, SCs, and FCs actually promote student understanding. Nevertheless, the data presented here on the conceptual difficulties students experience when attempting to learn these concepts suggest that any attempt to enrich a student's understanding of evolution with evo-devo content may ultimately fall short if that student does not already possess (or is not provided with) the basic tools of developmental biology.
ACKNOWLEDGMENTS
We thank the NESCent, which funded the EvoCI Toolkit working group, making this work possible, as well as other members of the working group, including consultant Kathleen Fisher. We also thank University of Wisconsin La Crosse faculty (Mark Sandheinrich, Nick Downey, Tisha King Heiden, Tim Gerber, Roger Haro, Anita Baines, Jennifer Miskowsky, and Eric Strauss) and Oklahoma State University faculty and staff (Karen McBee, Karen Smith, and Michi Tobler), who surveyed their biology courses to gather data on student concepts, and Katherine Moriarty, who transcribed student responses. Finally, we thank members of the EvoDevo ListServ for their anonymous contributions to our survey (and Pam Diggle and Billie Swalla for access to the listserv), as well as the many students at several institutions who contributed their time. G.K.D. is supported by the Elizabeth B. Jackson Fund at Bryn Mawr College and award IOS-1051643 from the National Science Foundation (NSF). NESCent is supported by award EF-0905606, also from the NSF. Any opinions, findings, conclusions, or recommendations expressed in this article are those of the authors and do not necessarily reflect the views of the NSF.
FOOTNOTES
†Present address: Department of Ecology and Evolutionary Biology, University of Kansas, Lawrence, KS 66045.



