Non-STEM Undergraduates Become Enthusiastic Phage-Hunters
Abstract
To increase science literacy and appreciation among nonscience majors, we offered a course in which 20 non-STEM (science, technology, engineering, math) undergraduates participated in a unique, two-semester research experience. Each student isolated and characterized his or her own bacteriophage from soil samples. One bacteriophage was selected for sequencing and together, the class annotated the genome of the newly sequenced bacteriophage. The class produced a group poster and gave PowerPoint presentations, and one student presented the joint work at a science symposium.
INTRODUCTION
The National Genomics Research Initiative (NGRI) is a program instituted and supported by the Howard Hughes Medical Institute's Science Education Alliance (HHMI-SEA) (for more information on the NGRI program, see www.hhmi.org/grants/sea). Its aim is to increase science literacy and to stimulate interest in biomedical research by engaging students in a genuine research experience. The NGRI course was modeled after ongoing work conducted by Dr. Graham Hatfull at the University of Pittsburgh (Hatfull et al., 2006). Twelve institutions initially offered the course beginning in fall 2008. Participating students collected soil samples from which they isolated viruses that infect mycobacteria (i.e., mycobacteriophage). They characterized their phage isolates on the basis of plaque morphology, DNA restriction digestion, and electron microscopy (EM). One phage from each institution was selected and sent to the Department of Energy's Joint Genome Institute for sequencing at the end of the first semester. In the following semester, students analyzed and annotated the resulting sequence and uploaded their completed product to GenBank, an online database of genetic sequences maintained by the National Center for Biotechnology Information (Benson et al., 2007). Funding for the NGRI continues for a total of 3 yr, after which nonmaterial support is offered to assist institutions in continuing the course on their own.
Eleven of the 12 institutions that offered the NGRI course limited participants primarily to undergraduate students who were either declared biology majors, or who had expressed an interest in becoming a biology major, or who were offered the NGRI course in place of their main introductory biology laboratory. We saw the NGRI course as an opportunity to positively influence mainly students who had up to this point in their academic careers demonstrated little or no prior interest in science. We felt that providing nonscience, non-STEM (science, technology, engineering, math) students an opportunity to carry out authentic research would allow them to experience the excitement and rewards inherent in scientific inquiry and exploration.
To attract nonscience majors, we designed our course, which we called “eBiology,” to satisfy the academic requirement that all graduates must have completed a science course having a lab component. We also stipulated that the course not satisfy any requirement for biology/biochemistry majors. Sixteen students (10 females, six males) from diverse ethnic backgrounds participated in the first semester. Nine were freshman and three were sophomores. Some of these students chose not to participate in the second semester for a variety of reported reasons, including course conflicts and graduation. In total, seven students participated in both semesters. In the second semester, we added an additional nine students of whom four were nonscience majors. Altogether, 25 ethnically diverse students participated: 17 females and eight males. Although the second semester entailed analyzing the data obtained in the first semester, there was no pedagogical reason to exclude students who had not completed the first semester. These students consisted of four biology majors and one biochemistry major and, presumably, had a stronger background than the nonmajor participants in the basic molecular biology used in the course. They were, however, relatively naïve with respect to bioinformatics, the specific topic of the course. Thus, we allowed nine new students to register: six upperclassmen and five STEM students. The addition of these savvy students served the course well. They had expressed an interest in bioinformatics and became an additional resource for the younger non-STEM students.
STALKING PHAGE SAMPLES DURING THE FIRST SEMESTER
Our class met twice each week for 3 h of lecture and lab time. The lab remained open and available for supervised work on two additional days for 2 h each, and additional time if it was requested by students. We found a remarkably high level of student participation outside of the formally scheduled meeting times, and frequently we had to extend the hours of lab availability. Each week began with a lecture covering the necessary theory and demonstrations of techniques the students would be using. We also incorporated weekly formal lab meetings during which students presented their progress and problems were discussed with the class.
Using a spatula and 15-ml sterile centrifuge tube, the students collected and tested two batches of five soil samples of their own choosing for the presence of mycobacteriophage. The sample was flooded with a buffer and the buffer was allowed to settle. Then, the supernatant was filtered through a 0.22-μm membrane and tested against the host organism Mycobacterium smegmatis (ATCC 700084; American Type Culture Collection, Manassas, VA). Students who were initially unable to find a phage enriched their soil sample by adding it to a mid-log culture of the host organism. The infected culture was incubated overnight with shaking. The next day, the medium was centrifuged, filtered, and then tested against the host bacterium with very high success. Ultimately, each student had a phage to investigate, most from soil isolations, although two students were allowed to work together to isolate and characterize an apparent lab contaminant. Students then obtained a pure phage population through repeated titrations. Once we were convinced a pure phage population had been obtained, each student harvested a high-titer lysate. The high-titer lysate was used for electron microscopy and to isolate DNA for restriction digestion analysis. All of the students prepared a grid for the transmission electron microscope; some were able to work with our electron microscopy facilities manager, but others left the grid with her for examination. All of our students succeeded in obtaining enough lysate to produce a micrograph of their phage (Figure 1and Supplemental Material), and all but one student were able to isolate enough phage DNA to examine the digestion pattern by agarose gel electrophoresis (Figure 2 and Supplemental Material). Phages were creatively named by the discovering student, and at the end of the semester each student gave a PowerPoint presentation describing his or her phage in detail. Two students had both sufficient quality and quantities of DNA to be candidates for sequencing. Based primarily on these two criteria mycobacteriophage Puhltonio was selected for sequencing. This particular phage name is a combination of the discovering student's last name and the student's mother's last name. The remaining phages have been archived by HHMI-SEA for future investigation.
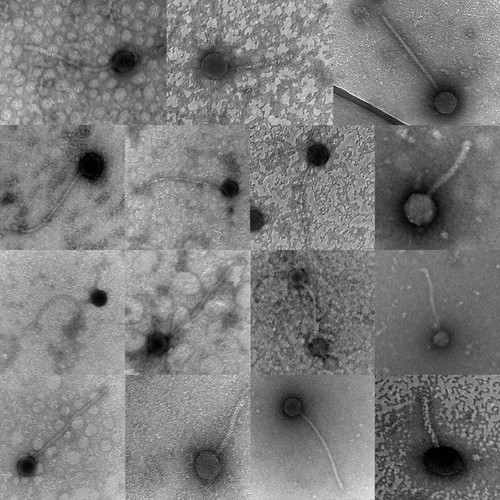
Figure 1. Transmission electron micrographs of mycobacteriophages isolated by students. See Supplemental Material for additional EM images.
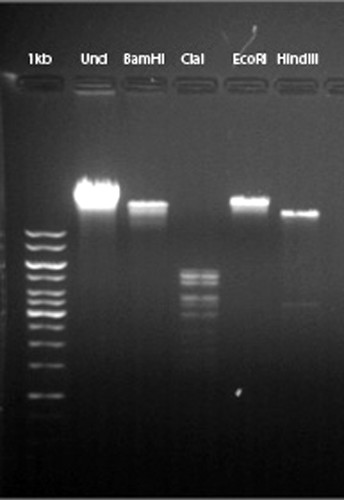
Figure 2. Example of student's digested phage DNA. This gel contains DNA from mycobacteriophage Puhltonio. Lanes 1 and 2 contain 1-kb standards and 0.5 μg of undigested phage DNA. Lanes 3–6 contain 0.5 μg of phage DNA digested with 10 U of the indicated restriction enzyme for approximately 2 h at 37°C. The gel is a 0.8% agarose submersible horizontal gel (7 × 10 cm) run in TAE buffer at 100 V and visualized with 0.5 μg/ml ethidium bromide. See Supplemental Material for additional gel images.
GENE IDENTIFICATION FOR THE SECOND SEMESTER
During the following semester, we met for 50 min three times a week in a dedicated computer lab for data analysis. All students had access to a computer with the required software preinstalled or were given access to the software to use on their laptops when possible. The approximately 68-kb genome was divided into eight equal regions and distributed among the 16 students so that each region could be analyzed by two students independently. Lectures were given as appropriate to familiarize students with the theory behind the procedures they were performing. The lecture topics early in the semester included concepts such as the central dogma, gene structure, and sequencing. Procedure-oriented lectures based on use of required software were presented immediately before starting any new analysis technique.
Students identified open reading frames as putative genes in their own region by using Genemark and Glimmer to look for typical prokaryotic gene landmarks such as translation start and stop codons, “promoters” and “Shine-Dalgarno sites.” Promoters are nucleotide sequences to which RNA polymerase binds to initiate transcription. Once RNA polymerase is bound to a promoter, transcription can proceed to produce a complimentary RNA “transcript” of the gene. Shine-Dalgarno sites are nucleotide sequences near the beginning of the RNA transcript where ribosomes bind so that the mRNA can be translated into protein. These sequences can often be found immediately before the translated portion of a gene. Students also compared their potential gene sequences against GenBank databases with the computer applications Basic Local Alignment Search Tool (BLAST)n and BLASTx to identify homologous sequences of known genes.
When each pair of students agreed on a putative map of their region, they presented their findings to the class for further discussion. After much discussion, the class agreed on the annotations of each region, and a full map was produced. Both individual genes and the entire resulting genome was further analyzed by students using Phamerator (Cresawn et al., 2007), which allowed students to visualize both a full graphical representation of the genome of the phage (Figure 3) and the relationship of Puhltonio to other mycobacteriophages.
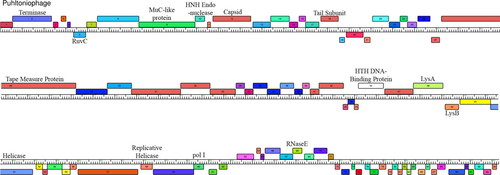
Figure 3. Genome map of Pultonio. Boxes represent putative genes. The numbers represent gene families (Phams) as determined by Phamerator. Labels indicate homology to known genes.
At the end of the second semester, each student pair gave a PowerPoint presentation of his or her work and explained how it fit with the rest of the annotated phage genome and with other mycobacteriophage genomes. The students also collectively produced a poster presenting the entire year's research. Finally, a single student was selected to present the results at the first HHMI-SEA NGRI symposium along with students from the other 11 institutions (Figure 4).

Figure 4. Rebecca Goldstein, an English and math major, presented at the First Annual HHMI-SEA NGRI symposium at HHMI's Janelia Farm Research Campus in Ashburn, VA.
STUDENT OUTCOMES
There is currently an ongoing debate in the education community concerning the merits, or possible lack thereof, of inquiry-based courses (Kirschner et al., 2006; Hmelo-Silver et al., 2007). We will not attempt to address this debate here, although it has been shown that inquiry or “problem-based” courses can increase the students' interest and participation in science courses (Krajcik et al., 1998). Also, recent work (Smith et al., 2009) demonstrates that peer discussion increases students' understanding of scientific concepts. The overriding objectives of eBiology were to increase students' content knowledge and laboratory skills and their appreciation of the process of science. To do this, we developed several subobjectives:
To learn basic phage and bacteria biology
To learn the central dogma of genetics
To learn microbiological lab techniques
Aseptic technique
Plaque assays
DNA extraction and characterization
Electron microscopy
To learn the theory of the bioinformatics tools used
To learn the landmarks of genes
Promotors
Shine-Dalgarno sites
Start codons
Stop codons
To learn use of data mining software
GeneMark and Glimmer
Apollo
BLAST
Phamerator
We can clearly assess the level of success of several of our goals. Our non-STEM students easily mastered the technical aspects of the course despite their prior lack of laboratory experience. Fifteen mycobacteriophage were isolated and characterized by electron microphotography and plaque morphology, 14 were characterized by DNA restriction analysis, and one phage, Puhltonio (named by Jake Puhl, the student who isolated it) was successfully sequenced and annotated and has been submitted to GenBank (accession GQ303264). The mastery of the theory was assessed by quizzes, exams, and the presentations each student gave to the rest of the class.
We have not yet measured the students' change in appreciation of science, although HHMI CURE and SURE survey instruments are in the process of being used to investigate just such effects across all of the participating institutions. We can report that we were pleasantly surprised by the gusto with which our nonmajors tackled the assignments, both in the lab where they spent many hours on data collection and on the computers from which they argued forcefully and coherently whether one particular gene or another was actually a “real” gene. We also can offer anecdotal evidence of a positive effect on students' attitude toward the process of scientific inquiry in the form of comments culled from students' anonymous written evaluations:
“[The best part was] … Getting to do real research (my phage is archived!).”
“The best part about this course was the opportunity to be a part of a real experiment … and for someone who had never used a Bunsen burner before, this was AMAZING.”
“It gave me insight and sparked an interest in a field I was previously indifferent to and did not enjoy.”
“This class was awesome. Makes me hate biology a lot less.”
In his presentation to the rest of the class, philosophy major David Tatum made this observation: “One of the main lessons learned throughout this process of discovery was the value of persistence in the face of a perversely uncooperative universe.”
These nonscience majors were clearly excited to be doing research. They were involved in decision making; they made decisions concerning protocols in the first semester and about which genes were real in the second semester. We will be tracking them to see whether there is any change in their major, which can be safely interpreted as a clear indication of a change in outlook. Whether this happens or not, we are convinced that nonscience majors' exposure to research will positively affect their perceptions when hearing about “wasted research dollars” and other negative, research-related articles in the popular press.
This two-semester-long class provided eBiology students with a unique experience that most engineering and science students never get, let alone nonscience majors. The course provides enough time in the lab to complete an authentic and inherently engaging experiment with all of the usual stumbles and setbacks investigators typically encounter along the way. The course project also yields a wealth of data that needs to be analyzed and made sense of. Students discover that discussion is important to the sense-making process and that science is collaborative, in contrast to the customary fictional image of scientists as solitary creatures who make new discoveries on their own. Argument and discussion, often a normal part of non-STEM courses, are here seen by students to be a normal part of “doing science.”
We find that this course is appropriate not just for students who are already interested in the sciences. We therefore believe that the framework of inquiry-based instruction in any biology course can be adapted for students in nonscience majors with an excellent outcome. It also may be possible to infer that similar problem-based courses in other STEM disciplines could be just as successful in helping nonscience majors appreciate the process of scientific discovery.
ACKNOWLEDGMENTS
We thank the following people for assistance in the presentation of eBiology: Chere Petty, Ralph Murphy, Colleen Wilkens, Tim Ford, and Phil Sokolove. Deborah Jacobs-Sera (University of Pittsburgh), Tuajuanda Jordan, Lucia Barker, Melvina Lewis, Matt Conte, Kevin Bradley, and Razi Khaja (all from HHMI) were invaluable resources. Support for this work was provided by the HHMI-SEA and the University of Maryland Baltimore County Honors College.



