Video Views and Reviews: Mitosis, Microfibers, and Motility
Here I review videos depicting various aspects of microtubule dynamics, from papers that were published in Journal of Cell Biology (JCB). The papers describe, respectively, changes in the activity of the G protein Rho-A during cell division (Yoshizaki et al., 2003), the dynamic behavior of microtubules attached to kinetochores (Maddox et al., 2003), and the targeting of microtubules to sites of focal adhesion in migrating fibroblasts (Krylyshkina et al., 2003). In preparing this review, I used the extensive archive of video material at the JCB's Web site entitled “Annotated Video Collection” (AVC; ( http://www.jcb.org/misc/annotatdvideo.shtml). I commend the editors of JCB for providing this valuable service, and I encourage editors of other journals that archive similar records to organize their collections in a similar fashion.
What makes the AVC especially valuable is evident in its title. AVC collates peer-reviewed research articles and their supplemental video clips conceptually by cellular topic and subtopic and provides a brief annotation for each video regarding content. Within each heading the articles are arranged in reverse chronological order, with the more recent articles appearing near the top of each listing. The annotations are brief summaries of research results and highlighted key words within each annotation provide hyperlinks to the complete article in JCB and to some of the supplemental videos. The annotations are terse and well written (apparently by a single individual), and occasionally the articles seem connected (if only by serial juxtaposition). Using AVC, Cell Biology Education readers and their students could locate videos of interest and, also, could organize journal club discussions or sections of advanced courses around the various topics or subtopics. Moreover, a link to the URL would be a useful addition to the Web page of any course interested in cell biology. AVC is current through the end of 2002 (Volume 159, Number 4, of JCB), which is probably the cutoff point for public access to the articles. I enthusiastically recommend AVC to readers of these reviews.
Again, I invite your comments on these reviews and your suggestions of other peer-reviewed videos for possible review as educational material.
BEHAVIOR OF RHO-FAMILY GTPASES DURING CELL DIVISION
Movies of cultured cells undergoing mitosis are fairly common, and after a while they all begin to look alike. As one who is especially jaded in this regard, however, I enthusiastically recommend this video and paper by Yoshizaki et al. (2003) for undergraduate study, for several reasons. First, as illustrated in Figure 1, the video is remarkable in that it consists of two different images of a HeLa cell in mitosis, and because the images were captured in a quickly alternating fashion, the video provides two different kinds of information about the process. The left-hand images were obtained by differential interference contrast microscopy (DIC), and they clearly depict the various stages and details of cell division, beginning with a single cell in interphase and ending with two daughter cells also in interphase. The right-hand images, in contrast, document the changes in fluorescence associated with activation and inactivation of the regulatory G protein Rho A within the same cell(s). The right-hand sequences also well illustrate the usefulness of fluorescence resonance energy transfer (FRET) as a means for monitoring protein-protein interactions or, as in this example, the interaction of two different domains on the same protein. The video contains a lot of visually correlated information, about the structural features of mitosis and its functional regulation, and that's another reason I like it. Finally, I recommend the sequence because when projected at 15 frames per second (and speeded up 1800 times), events unfold in a visually dramatic and memorable fashion.
The video is technically well done, and it presents something useful for audiences at different educational levels. For more superficial coverage, introductory students viewing the movie can appreciate the continuity of the cell cycle from interphase through mitosis and cell division and move beyond any static impressions of the process they may have gained from “stages of mitosis” pedagogy. They can also glimpse the importance of correlated changes in G protein structure during cell division, without understanding the details of either DIC or FRET and without prior knowledge of G protein function. Given the high resolution associated with DIC, more advanced students can follow the process in greater spatial and temporal detail if they slow down the projection rate or step through the images frame by frame. To appreciate how Rho A function is correlated with cell division, they will need to understand that for the FRET studies, the authors developed a chimeric probe—“Raichu”—that contained RBD (a Rho A binding domain), a truncated Rho A fragment (which has a GTP/GDP binding domain), and, at either end, yellow fluorescent protein (YFP) and cyan fluorescent protein (CFP). When Rho is inactive and bound to GDP, the chimera is structurally extended and excitation at 433 nm produces blue CFP fluorescence (475 nm). When activated, Rho A exchanges GTP for GDP and binds with RBD, and the chimera folds, bringing CFP and YFP into close proximity: so close that excitation energy absorbed by CFP is transferred to YFP and released as green fluorescence (e.g., Lodish et al., 2003, Fig. 13-12, and Yoshizaki et al., 2003, Fig. 1). When excited at 433 nm, the “inactive” Rho A chimera appears blue, while the folded, “active” form appears green. The colors shown in Figure 1 (and the videos), however, are somewhat confusing because they represent the ratio of YFP-to-CFP fluorescence and not the fluorescence itself. Thus, the image colors range from blue (low YFP fluorescence) to red (high YFP fluorescence), and instances of efficient energy transfer representing activated Rho A appear red (not green), while inactive Rho A appears blue.
Figure 1. Activity of the G protein Rho A localized at the cell surface of dividing HeLa cells during G2 of prophase (A, B), metaphase (C, D) and telophase (E, F), observed alternately by differential interference contrast microscopy (A, C, E) and epifluorescence microscopy (B, D, E). Note the presence of condensed chromosomes at the metaphase midline (C) and at both telophase poles (E). Colors in B, D, and E represent the transfer of resonance energy (FRET) within a chimeric protein consisting of a truncated form of Rho A and a RBD linked with YGP at one end and CGP at the other. The videos were colored to reflect the ratio of YFP-to-CFP emissions, ranging from blue (low ratio) to red (high ratio). Blue regions contain inactive Rho A chimera; red regions, folded and activated chimera. The video is located at http://www.jcb.org/cgi/content/full/jcb.200212049/DC1/1. Reproduced from Journal of Cell Biology, Vol. 162, No. 2, 2003, pp. 223-232, Video 1, by permission of the Rockefeller University Press.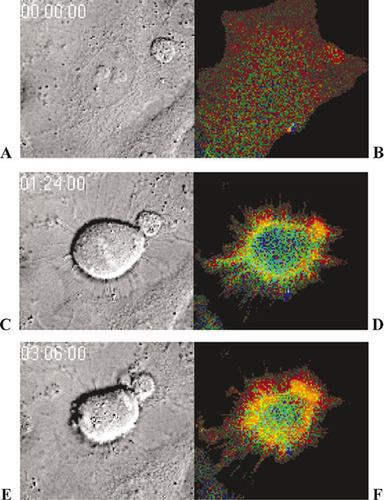
Following an introduction to FRET, students can also explore the cyclical inactivation and reactivation of Rho A as interphase alternates with mitosis and cytokinesis, and they can begin to see the cellular events in their biochemical context. Since the paper also presents similar data for other Rho-family G proteins and their regulators, study can be expanded to include other possible regulatory mechanisms on mitosis and possible interactions among the various factors. Finally, students with more of a molecular bent can use the paper and some of its citations to explore the creation and testing of genetically encoded Raichu probes of G proteins. This paper is a nice, varied teaching resource.
MOVEMENT OF KINETOCHORES AND MICROTUBULES DURING MITOSIS
The motility of mitosis and microtubule dynamics are explored in a recent paper by Maddox et al. (2003). These scientists used high-resolution confocal fluorescence microscopy to document the events occurring at the centromere during metaphase and anaphase in mitotic spindles isolated from eggs of the South African frog, Xenopus laevis. To appreciate the results of this study and their significance, however, some background material and terminology are reviewed in the following three paragraphs.
Most students know the mitotic spindle forms during early prophase by microtubule growth outward from two poles (centrosomes). During spindle formation, duplicated chromosomes condense, remaining attached at a common centromere, and the nuclear envelope disappears. Those elongating microtubules that contact the centromere become attached at a specialized protein structure called a kinetochore and are called kinetochore tubules. Several tubules attached to the same kinetochore coalesce to form a kinetochore fiber. The kinetochores, in turn, are thought to “cap” the growing () ends of the anchored tubules. Paired chromosomes with+kinetochore fibers extending toward the opposing centrosomes are effectively “captured” within the spindle, which is formed by other, parallel (or polar) microtubules that have grown from pole to pole and are not attached to kinetochores (see, e.g., Lodish et al., 2003). Tubular bundles or fibers are the spindle units visible by light microscopy.
While many students may appreciate the structure and the origin of the spindle, as presented above, some, especially introductory students, may also equate the “finished” product with the static images they have seen in texts or histological material. They may only vaguely be aware that the spindle, including both kinetochore and polar tubules and fibers, is a dynamic structure. Microtubules, in fact, are steady-state organelles: that is, at times they may appear to be constant in length because assembly at the (-) ends is balanced more or less by disassembly at the+ () ends located in the centrosome. Such steady-state behavior was deduced from different experiments and observations, most recently from viewing the poleward movement of spots or “speckles” of labeled subunits (tubulin) in metaphase tubules that for the most part were unlabeled (Mitchison and Salmon, 2001).
A spindle consisting of speckled microtubules looks as if it has measles, and when examined during metaphase in living material, individual measles spots (speckles) were not static. They moved poleward at similar velocities, although the spindle fibers themselves appeared to be quite stable and constant in length (see also Lodish et al., 2003). Most students will appreciate the simplest explanation of such movement, which entails tubulin subunits“ treadmilling” from their point of assembly at the (+) ends of microtubules to the (-) ends where disassembly occurs. In kinetochore tubules, treadmilling occurs from the kinetochore towards the centrosome.
In the present report, Maddox et al. (2003) extend the collaborative effort that produced the 2001 study by Mitchison and Salmon to include measurements of the anaphase movements toward the poles of both labeled kinetochores and tubule speckles. In isolated egg spindle preparations, kinetochores were labeled with red fluorescent antibodies prepared against the kinetochore protein CENPH-A and green-speckled microtubules were created by introducing a substoichometric “pulse” of X-rhodamine-labeled tubulin.
Figure 2 summarizes the behavior of kinetochores and spindle microtubules at roughly the beginning (Figure 2A), the middle (Figure 2B), and the end of anaphase (Figure 2C) of anaphase. While colorful, they do not do adequate justice to the vivid impressions conveyed by the time-lapse video (Maddox et al., 2003, Video 3), which should be examined closely and viewed repetitively. In the video, students will note that speckles, as well as kinetochores, move poleward as chromosomes separate during anaphase, and perhaps they can also detect differences in the respective rates of movement. Data from this video (summarized in Maddox et al., 2003, Fig. 3) indicate that speckles usually moved at different rates than did their respective kinetochores—sometimes more slowly, sometimes more rapidly—but only rarely did a kinetochore and the speckles of its attached tubules move at the same velocity. This is an unexpected result and one likely to puzzle most students (and many of their teachers), and the quantitative reduction of these data presented by Maddox et al. 2003, (Fig. 3) is not easy to follow. Their discussion and explanatory models (Fig. 1) are quite helpful, however. When dissecting this paper with intermediate or advanced students, I suggest it would be appropriate to view the video several times and then to try tracking the behavior of specific kinetochores and their respective tubule speckles. Then it would be useful to explain how the velocities of single kinetochores and their respective tubule speckles were obtained from kymograph traces along single fibers, using the Mitchison and Salmon (2001) paper and Figure 20-38 of Lodish et al. (2003) as aids. Following these steps, students should be in a stronger position to appreciate the authors' three models for the various movements (Maddox et al., 2003, Fig. 1).
Reviewing the movie, most students will also appreciate that the egg spindle apparently expands in length and girth during anaphase, accompanied by an overall decrease in speckling fluorescence. Exploration of these changes leads naturally to an examination of the two different modes of chromosomal separation, which are called Anaphase A and Anaphase B (see, e.g., Lodish et al., 2003), and to a discussion of the relative importance of, respectively, treadmilling and sliding mechanisms of microtubular motility. The more critical students also may wonder whether these changes in spindle dimensions also affect the data. More advanced students may wish to examine refinements of the model (Maddox et al., 2003, Fig. 4), especially as related to the importance of kinetochore tension through centromere stretching. They may also wish to discuss whether some aspects of the data might reflect the isolation and in vitro labeling and observation conditions and how the most likely model might be more rigorously tested in vivo. Some might wonder whether similar results would be obtained if speckles could be produced in spindle isolates using substoichometric concentrations of green fluorescence protein (GFP)-tubulin.
TARGETING OF MICROTUBULES TO FOCAL ADHESIONS
Over the past decade, the dual motility roles played by sliding microfilaments and microtubules, and by their assembly/disassembly, has become increasingly evident. Neither behavior, however, can produce directional locomotion in unattached cells (except, of course, in those having flagella or cilia). Correspondingly, it is also important to understand how dynamic microfilaments and microtubules are anchored to the substratum through the intermediacy of integral membrane proteins in the plasma membrane of moving cells.
Figure 2. Confocal fluorescent images showing the behavior of chromosomal kinetochores (red) during anaphase relative to the poleward flux of microtubules (green), at 0 s (A) and approximately 125 s (B) and 400 s (C) after filming began. Microtubules and kinetochores were labeled, respectively, with X-rhodamine and an antibody against CENP-A that had been tagged with ALEXA 488 NHS. Images were acquired at intervals of 5 s. The video is located at http://www.jcb.org/cgi/content/full/jcb.200301088/DC1/5. Reproduced from Journal of Cell Biology, Vol. 162, No. 3, 2003, pp. 377-382, Video 3, by permission of the Rockefeller University Press.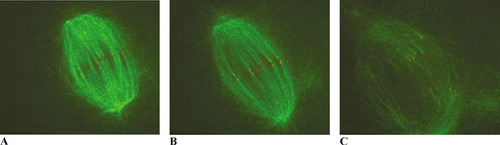
Figure 3. Confocal fluorescent images of microtubule tips (green) polymerizing toward focal adhesions (red) in a cultured line (CAR) of goldfish fin fibroblast, taken at the beginning of the film (A), at 2 s (B), and at 6 s (C). The growing (+) ends of microtubules are labeled with a chimeric protein containing CLIP-170 (a microtubular tip protein) and GFP. Focal adhesions are labeled with a chimeric protein (DsRed-zyxin) often found in substratum adhesion sites. The real sampling time is shown in seconds, and the interval between video frames is 2 s. The video is located at http://www.jcb.org/cgi/content/full/jcb.200301102/DC1/5. Reproduced from Journal of Cell Biology, Vol. 161, No. 5, 2003, pp. 853-859, Video 5, by permission of the Rockefeller University Press.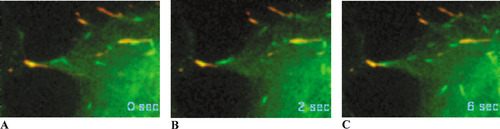
In their brief report, Krylyshkina et al. (2003) describe the dynamic behavior of microtubules polymerizing near focal adhesions in goldfish fin fibroblasts doubly transfected with genes for two chimeric proteins, GFP-CLIP-170 and DsRed-zyxin. The polymerizing (+) tips of microtubules were marked by the presence of CLIP-170, a microtubule cross-linking protein with a Mr of 170 kDa, joined with GFP (cf. Schroer, 2001). Focal adhesions were identified through the presence of zyxin, an ancillary adhesion protein, labeled with DsRed. The movement or putative growth of green microtubule tips into red adhesion sites was then documented by video fluorescence microscopy. Several confocal images obtained over a 6 s interval and abstracted from a longer dual-color sequence (Supplemental Video 5) are presented in Figure 3.
The apparent targeting of growing microtubules toward a single focal adhesion site is evident in this very striking video sequence, and the close proximity of the green organelles and the red adhesion site is suggested by the yellow spot within the adhesion site and by the very thin focal plane obtained with confocal imaging. Observant students will note that while numerous microtubule tips sequentially enter a single adhesion site and the center of the adhesion site “flickers” over time, the yellow spot does not seem to increase in size or intensity. The more curious and vocal will want to know why not—that is, how the center maintains a steady-state association of adhesive integral membrane proteins and tubular tips—and a good discussion of microtubule dynamics and the optics of confocal imaging can ensue. To address this question further, more advanced students may wish to examine the videos produced by the other imaging technique employed by the authors—total internal reflection fluorescence microscopy, or TIRFM. These data support the confocal observations summarized in Figure 1, but the optics of TIRFM seem too technical for most undergraduate audiences.
Other students may question whether the tips are moving only by microtubular growth—that is, through assembly at their (+) ends—or whether entire organelles might be moving as the result of CLIP-coated tips sliding forward. Possibly, both modes of motility are involved. Addressing this concern will require close reading of Krylyshkina et al. (2003) and further work in the literature (e.g., Schuyler and Pellman, 2001), and an interesting journal club discussion could be developed around this point. It is also interesting to ask, as have Krylyshkina et al., how growing microtubules could be directed or targeted to focal adhesions. The mechanistic model presented in their Figure 5 is especially clear and thoughtful in addressing this matter. Discussion could also be generated toward how the model might be tested experimentally (and the short report expanded into a full length research article). Finally, some students may want to know how such dynamic intracellular behavior relates to cell locomotion, since none of the cells imaged in the eight video sequences archived with this paper seems to be moving across the substratum. In extending their investigation of microtubule targeting and fibroblast motility, students may find an earlier review of microfilament dynamics in lamellipodia helpful (Small et al., 2002). All in all, the paper by Krylyshkina et al. (2003) presents a striking set of video records and a provocative study.



