General Chemistry: Expanding the Learning Outcomes and Promoting Interdisciplinary Connections through the Use of a Semester-long Project
Abstract
The laboratory component of a first-semester general chemistry course for science majors is described. The laboratory involves a semester-long project undertaken in a small-group format. Students are asked to examine whether plants grown in soil contaminated with lead take up more lead than those grown in uncontaminated soil. They are also asked to examine whether the acidity of the rainwater affects the amount of lead taken up by the plants. Groups are then given considerable independence in the design and implementation of the experiment. Once the seeds are planted, which takes about 4 wk into the term, several shorter experiments are integrated in before it is time to harvest and analyze the plants. The use of a project and small working groups allows for the development of a broader range of learning outcomes than occurs in a“ traditional” general chemistry laboratory. The nature of these outcomes and some of the student responses to the laboratory experience are described. This particular project also works well at demonstrating the connections among chemistry, biology, geology, and environmental studies.
INTRODUCTION
Recent reports and initiatives such as Shaping the Future (National Science Foundation, 1996), Science Teaching Reconsidered (National Acadamies Press, 1997), Reinventing Undergraduate Education (Carnegie Foundation, 1998; the Boyer Report), Project 2061 (American Association for the Advancement of Science, 1985), and BIO 2010 (National Academies Press, 2003) have advocated an expansion of the learning goals for the undergraduate curriculum and the use of different methods of instruction than traditionally used in science courses. These reports generally recommend involving science students in activities such as investigations as a way of gaining a fuller appreciation of the nature of science and better developing the broad range of skills necessary for successful careers. In a report aimed at assessment of undergraduate education, the following learning outcomes were described (Ewell, 2001).
Knowledge Outcomes
Knowledge outcomes are “particular areas of disciplinary or professional content that students can recall, relate, and appropriately deploy.”
Skills Outcomes
Skills outcomes are “the learned capacity to do something —for example, think critically, communicate effectively, productively collaborate, or perform particular technical procedures—as either an end in itself or as a prerequisite for further development.”
Affective Outcomes
Affective outcomes “usually involve changes in beliefs or in the development of particular values, for example, empathy, ethical behavior, self-respect, or respect for others.”
Learned Abilities
Learned abilities “typically involve the integration of knowledge, skills, and attitudes in complex ways that require multiple elements of learning. Examples embrace leadership, team-work, effective problem solving, and reflective practice.”
I believe that these learning outcomes provide an excellent summary of what ought to be the goals of an undergraduate education. Furthermore, there is a growing awareness of and emphasis on the importance of incorporating aspects of interdisciplinarity into the undergraduate curriculum. Many of the scientific problems investigated today are exceedingly complex such that multidisciplinary teams are required for success. Learning aspects of other disciplines and learning how to talk to practitioners of other disciplines are important skills, and skills that ought to be part of an undergraduate education in the sciences.
A “traditional” undergraduate chemistry curriculum, which tends to incorporate individual rather than group work and emphasize content areas in the classroom and the coverage of techniques and development of manipulative skills in the laboratory, only addresses some of the knowledge and skills outcomes described above. Through proper design, it is possible to achieve a much broader range of learning outcomes and demonstrate the connection between chemistry and other disciplines in an undergraduate chemistry curriculum. A science curriculum that achieves the variety of learning outcomes described above must incorporate investigations and collaborative activities among students. To improve the effectiveness of such a curriculum, these types of activities should be started at the introductory level, be reinforced through upper-level courses, and culminate in a capstone research experience.
I describe the laboratory component of the first semester of a two-semester-long general chemistry course that we have offered at Bates College since 1998 that relates the fundamentals of chemistry to the study of the environment. The course sequence counts for the chemistry major and satisfies the general chemistry prerequisite for all upper-level chemistry courses. Much of the classroom portion of the course involves cooperative learning, and readers interested in more information about the course topics and cooperative in-class activities are referred to a previous article (Wenzel, 2001) and to materials available on my Web site at http://www.bates.edu/x50814.xml#Tom.
A maximum of 60 students are in the class, and there are three laboratory sections of no more than 20 students. To date, the course has been offered seven times and been taken by ∼400 students. Each laboratory section is taught by a faculty member and an undergraduate teaching assistant. About two-thirds of the students are in their first semester of college and have not yet declared a major. The remaining students are primarily upper-level biology, geology, and environmental studies majors. However, each year several students majoring in a humanities or social science discipline take the course to fulfill general education science requirements of the college. The text is the same text used for the other sections of general chemistry, and students can switch into or out of the thematic sequence between the first and second semester. The environmental topics incorporated into the course are augmented with reading material from a variety of sources. The laboratory includes a semester-long project that allows the students to conduct a research-like investigation. The project is also designed to show the connection of chemistry to biology, geology, and environmental studies.
GOALS AND OBJECTIVES OF THE LABORATORY EXPERIENCE
Because the course serves as the general chemistry prerequisite for all upper-level chemistry courses, it is essential even with a project that students gain a thorough experience with fundamental laboratory skills. These skills include learning how to weigh samples, use volumetric glassware, prepare solutions, and perform dilutions. They need experience with basic instrumentation such as a pH meter and spectrophotometer, and they must understand how to construct a standard curve. They need to analyze data and perform some fundamental aspects of statistical analysis, including the calculation of averages and standard deviations as well as assessing whether it is statistically valid to reject a data point. They must also appreciate the basic safety rules that apply to work done in a chemistry laboratory. Another goal of the laboratory is to provide the students with a fundamental understanding of stoichiometry and to then apply some aspects of stoichiometry in the execution of the experiments and the project. The goals just described were common to the introductory laboratory experience that characterized my course before I instituted the semester-long project, although in the prior format the skills were not really learned in as meaningful a context. Also, there was a tendency to provide many of the chemical solutions in the prior format, whereas with the project, each group must prepare everything they will use. As a result, the students have more opportunities to develop fundamental laboratory skills in executing the project.
Other goals and objectives go beyond those that were included in my prior structure of the introductory laboratory experience. I now want the students to practice oral and written communication skills and to have to think critically in the design of an experiment and evaluation of the data that arise from the experiment. The students are to gain experience in undertaking an investigation that is characterized by uncertainty and where neither they nor I know the answer beforehand. The students are also to gain experience in collaborating with others in a team approach to an investigation. Working in groups provides more potential for the students to develop respect for others and self-respect for their own accomplishments than can occur through individual work. The group nature of the project also provides the opportunity for students to exhibit and practice leadership skills. Analysis of the data provides the opportunity for reflection and for discussion of ethical practices. Instituting the project has provided the opportunity for students to practice a broader skill set. A peer and self-evaluation that will be described later allows me to assess in a formative and summative way certain aspects of student performance.
FACILITATING COOPERATIVE LEARNING
Prior research has shown that an instructor using cooperative learning must take an active role in facilitating effective group practices (Johnson et al., 1991). This role includes being explicit about the format and expectations of the cooperative learning activities and being ready to intervene if a group is not working well together. Most students have very little experience with cooperative learning and need a thorough discussion of the methods that will be used. I spend almost the entire first class explaining why I use cooperative learning, describing the specific procedures we will use, and laying out my expectations for the students when participating in cooperative learning activities. I go over these again in the first meeting with each laboratory section and reiterate them periodically throughout the term. I stress the importance of students being respectful of the thoughts and ideas of all of their group members. I emphasize that, for cooperative learning to be effective, a student who understands a concept must be willing to explain it to group members who do not. Furthermore, I emphasize that everyone must be willing to accept explanations from other members of their group.
As the term develops, I make a point of praising groups that are functioning well together and specifically identify what it is that I like about their performance. I readily intervene with a group when I see problems developing. If the problem resides with one individual, I meet with that person privately to discuss my concerns. If the entire group is performing poorly, I talk with the group to explain what I expect from them to function more effectively. A peer and self-assessment instrument is used in both a formative and summative way to improve and grade group performance.
LABORATORY EXPERIENCE
Using information gathered on the first day of class, I divide the students in each laboratory section into five groups of four. These groups are made as heterogeneously as possible with regard to gender, race, year of study, and major. Each group checks into a locker and then undertakes the cookie experiment, which is designed to demonstrate the different levels of uncertainty that can exist when performing measurements. Students are asked to determine 1) whether Double Stuff Oreo cookies really have double the“ stuff” and 2) the percentage by weight of chocolate in a chocolate chip cookie. Each student gets one of each cookie, so the groups have four data points to average and calculate a SD. Students are left to their own devices in determining how best to perform the separations and measurements. With the chocolate chip cookies, most students try to physically separate the chips from the batter by using a metal spatula, although every year some students try to perform the separation by immersing the cookies in water first. The measurement on the Oreo cookies is more precise (Table 1), although students realize that it is impossible to completely separate all of the filling from the cookie wafers such that small specks of wafer remain imbedded in the“ stuff,” and some of the “stuff” is still adhered to the wafer. The students find that the precision with the chocolate chip cookie measurement is far worse (Table 1), and in a group report are asked, among other things, to compare and comment on the precision of the measurements and sources of error. All of the groups appreciate that the separation of the chocolate chips from the cookie has much greater error than separating the “stuff” from the wafers and recognize that as one source of the difference in precision. Most of the groups also realize that the distribution of chocolate chips in cookie batter is more heterogeneous than the machines that likely squirt the“ stuff” into an Oreo cookie, such that the measurements of the percentage of chips in several chocolate chip cookies ought to show worse precision.
| Regular Oreo (g) | Double Stuff Oreo (g) | Chocolate chip (%) |
|---|---|---|
| 3.08 ± 0.23 (7.46) | 6.65 ± 0.24 (3.61) | 25.4 ± 6.4 (25.2) |
| 3.17 ± 0.09 (2.84) | 6.76 ± 0.18 (2.66) | 24.6 ± 2.9 (11.8) |
| 2.99 ± 0.13 (4.34) | 5.97 ± 0.13 (2.18) | 24.2 ± 3.7 (15.2) |
In the second week, the students are presented with the two questions they will examine through the semester-long project. The first question is whether plants grown in soil contaminated with lead take up more lead than plants grown in uncontaminated soil. We hypothesize that plants grown in contaminated soil are likely to have higher levels of lead. The second question is whether the lead uptake by plants varies with the acidity of the rainwater. In other words, does acid rain influence lead uptake? I describe how laboratory studies show that lead salts become more soluble in more acidic solutions. So long as the increased acidity does not affect the plant's mechanism for taking up lead, we can hypothesize that having more lead dissolved in the water would likely increase the level of lead in the plants.
The groups are then asked to generate a list of information they will need to know to undertake the project, variables they will need to adjust, and questions they may need to consider to complete the project. Using overhead transparencies, we generate a composite list for each laboratory section, and I type an overall list for the entire class and distribute it to all the students. This list includes, among other things, what plants to grow, what soil to use, how much lead and what lead species to add to contaminate the soil, the chemical species and their concentration that make up acid rain, how to prepare acid rain, how and in what to plant the beds, what watering schedule to use, how to design a control, and how to analyze for lead. Students also realize that some variables are within our control but that others (e.g., light, humidity, temperature, and initial lead levels in the soil) are likely beyond our control.
The groups are then provided with a two-page list of seeds from a biological supply firm (Connecticut Valley Biological, Southampton, MA), and each group decides what plant species to grow. These selections, which usually include vegetables such as peas, beans, and tomatoes as well as flowering plants such as marigolds and zinnias, are ordered in time to arrive for planting, which will occur in 2 wk. After each group has reported what plant species they want to grow, which I list on the board, I ask them to consider what we might be likely to select if the course was being taught at a college located in Southeast Asia. Invariably the students answer “rice,” and then realize that rice was not even a choice from Connecticut Valley Biological. In a small way, they begin to appreciate that the social context within which we work may often influence the scientific investigations we undertake.
The students are next given a tour of the greenhouse to see the space that we have been allocated for our project. Many groups are surprised at how little space can actually be allocated to each group and have to scale back the ambitious plans they had in mind before seeing the facilities. The staff person who oversees the operation of the greenhouse shows them the different potting options that exist (flat containers to various sized pots) and goes over the rules for their use of the facility. The students realize that they will need to design a system to isolate each different plant sample to avoid cross-contamination of watering solutions (they usually decide to accomplish this by crafting aluminum foil containers lined with plastic wrap to hold each sample). They begin to appreciate the influence that time, space, and money can have on scientific investigations.
To prepare their watering solutions and contaminated soil, the latter of which is done using lead acetate (in early iterations we used leaded paint dust but this became prohibitively expensive), the students need an understanding of stoichiometry. Two cooperative learning activities are used in the second and third week of the laboratory to develop basic concepts of stoichiometry. Students complete these in their groups, have out-of-class assignments that they are to work on in their groups, and are given a quiz on stoichiometry in the laboratory. With this knowledge, they are then able to calculate how to prepare the contaminated soil (using lead acetate as the source of lead contamination) and acid rain solutions (starting with concentrated nitric and sulfuric acid).
Although students work cooperatively to learn the concepts in my classes, all of the exams are given as individual exercises so that I can assess each student's learning. Before instituting cooperative learning, <15% of the students in my introductory class got a grade of 95% or higher on the stoichiometry quiz. Since instituting cooperative learning, ∼60% of the students have gotten a grade of 95% or higher. Similarly, on the final for an upper-level course that involves the interpretation of NMR spectra, fewer than 10% of the students got all 10 of the problems correct before I began using cooperative learning. Since instituting cooperative learning, 45% of the students who have taken the course correctly answered all 10 of the problems. I teach an upperlevel analytical chemistry course that includes a unit on chemical equilibrium. By the end of this unit, we examine extremely complex systems with many simultaneous reactions. Before instituting cooperative learning, it was common to have a few students in the class make essentially no progress in understanding these complex problems such that they would often score in the single digits on a 100-point exam. As a result, class averages in the 45% range were common. Since instituting cooperative learning, single-digit scores no longer occur, and class averages are typically in the 65% range for exams on these exceedingly complex problems. The improvements I have seen since instituting cooperative learning are consistent with a large body of research that unequivocally demonstrates that cooperative learning leads to statistically significant improvements in academic achievement (Johnson et al., 1991).
In the third week, after completing the second stoichiometry problem set, the groups use the composite list of questions and variables generated by the class to help them develop their specific plan. They decide on a soil to use, a lead concentration for contaminated soil (I point out that the Environmental Protection Agency considers soil with lead levels above 400 ppm as in need of remediation, and groups have typically used levels between 500 and 1000 ppm), the exact nature of their watering solutions (acid composition and pH), and what containers they will use for the plants. Every year, we have had quite a range of plant species and particular conditions (lead concentration and acid composition) used by the different groups. When I first started the project, the students used soil they gathered from the local area; however, this presented certain problems. Some of the soil was so low in nutrients that the plants did not grow well. Other soils came from sites that were already contaminated with lead (we analyzed the lead level in the soil later in the term, so they only found this out after planting) such that some groups'“ uncontaminated” soil was similar in lead levels to other groups' contaminated soil. Finally, some students gathered soil from inappropriate places (the flower gardens at the president's house or one of the varsity athletic fields), so I now strongly urge them to use the greenhouse soil. The soil used in the greenhouse is a commercially available material known as“ Promix–compressed grow medium” that we purchase locally.
I provide a brief overview on the composition of acid rain. The groups have the option of using one or two acidic pH values relative to the control (the control is either tap or distilled water, which they choose) and of using all nitric, all sulfuric, or a mixture of nitric and sulfuric acid to prepare acidified watering solutions. We also discuss how they may want to use a more acidic pH than found naturally (i.e., pH 3) to possibly enhance the odds of seeing a trend with acidity. Most groups choose to use a 50:50 mixture of nitric and sulfuric acid to prepare acid rain, which is a reasonable approximation of the composition of acid rain in Maine. Some do use only one of the acids. Typical pH values for their rainwater range from 3 to 5. The last task we do in the third week is an exercise in which each group determines an exact recipe for preparing their watering solutions and contaminated soil. Preparation of the acidic watering solutions requires a two-step serial dilution. The more concentrated stock solution is prepared, and they dilute it with water as needed to prepare the watering solutions. These dilutions are written on the board and compared so that we are sure that each group has correctly performed the calculations.
In week 4, I provide them with thorough instructions on the use of pipettes and volumetric flasks. They then prepare their stock acid solution(s) and first sets of watering solutions, prepare the contaminated soil, fill their containers with soil, plant the seeds, set the containers up in the green-house, and devise a watering schedule. Watering presents an interesting facet of the project because the students soon realize how quickly the soil dries out in the greenhouse, which means that they need to come in off days, including weekends, to water. A special challenge is the 5-d break we have in October, because most of the students are planning to leave campus. Groups usually end up recruiting fall athletes or international students in the course who often are on campus over the break. The groups then monitor their plants and in the event that a sample does not grow, they either replant if time permits or share a sample with another group that had more than enough growth. Using the greenhouse soil has significantly reduced the number of groups that do not get sufficient plant growth, although occasionally a group has a sensitive species or overwaters their plants. Because there have always been other groups with plenty to share, replicate workup and analysis on a single sample is presented as a valuable thing for us to be doing to examine the level of precision among groups.
INTERVENING WEEKS TO HARVEST
During the period the students are watering and monitoring the plants (five laboratory sessions given the length of our semester), other shorter experiments are integrated into the laboratory. Because two of the experiments use sophisticated equipment for chromatographic analysis (ion chromatography and gas chromatography–mass spectrometry), the topic of chromatography is introduced through the use of paper chromatographic analysis of ink from felt tip pens. After analyzing three different brands of black pens, students are given a few small, cut-up pieces of a document written in one of the three pens (there are three different unknowns so not every group has the same answer) and must identify which pen was used to write the document.
Two of the other experiments have a direct relationship to the project. One experiment is that we set rain gauges on a roof outside the laboratory and collect rainfall over the term. The concentration of nitric and sulfuric acid in the rain is determined by measuring the pH and by determining the concentration of nitrate and sulfate by ion chromatography. The students can then compare actual acid rain to their own watering solutions and can see whether meteorological effects influence the makeup of acid rain. For example, Figure 1 shows the ion chromatographs for three rain samples. The sample from a storm that came in off the ocean only shows sulfate (Figure 1b), whereas storms from the west that travel over land have both nitrate and sulfate (Figure 1, a and c). Although storms that come from the east are relatively uncommon, low nitrate in such storms has been a consistent observation over several years of sampling. Because nitric acid is known to primarily come from anthropogenic sources such as automobile exhaust and power plant emissions, the low levels of nitric acid in storms coming in from the east is not surprising. Also, the rain sample on October 19, 2000 (Figure 1a) had more sulfuric than nitric acid, whereas the opposite was observed for the rain sample on November 9, 2000 (Figure 1c). The results from all of the samples collected over the term are compiled and provided to the students. They are required to incorporate a discussion of these measurements into their final written project report, comparing what was measured in actual rainwater with their watering solutions and commenting on any obvious trends of the amounts with respect to meteorological conditions.
The rainwater samples must be filtered through a 0.45-μm filter before injection into the ion chromatograph. The first year, using a nylon filter, we obtained chromatographs with no nitrate or sulfate ions but with a peak at a retention time consistent with the presence of acetate ion. An analysis of the exact same sample unfiltered provided a chromatogram with nitrate and sulfate ions and no peak at the retention time of acetate. Apparently, the nylon filters we purchased had trace amounts of acetate ion that underwent an ion exchange with the nitrate and sulfate in the rainwater samples. Filters made from cellulose do not reduce the nitrate and sulfate levels in the rainwater. We now use this example to show the students the level of care and verification that ought to accompany the analysis of trace-level constituents.
The second experiment related to the project involves the leaching of iron from an iron-rich schist. One goal is to demonstrate the basis for our hypothesis that increased acidity is likely to lead to higher concentrations of metal ions in water percolating through soil. We get the rock specimen from the geology department, and the rock is pulverized using a hammer. One-gram samples of the rock are then allowed to sit for a week in 10 ml of each group's stock (used as an especially acidic sample) and watering solutions, including the control. The rock is removed by filtration, and the aqueous samples are analyzed for their iron content using a classic spectrophotometric method based on the iron complex with 1,10-phenanthroline. This laboratory experiment gives the students further practice in using volumetric glassware and provides an understanding of the basis of Beer's law and spectrophotometry. Groups routinely find that the most acidic sample has the highest amount of iron. Interestingly, the distilled water sample used as a control also has a fairly high level of iron, and measurement of the pH of the final solution shows that the sample has become reasonably acidic. Presumably the rock itself is somewhat acidic, which accounts for the change in pH. Nevertheless, the students see a clear trend consistent with the hypothesis that increased acidity should enhance the leaching of metals from soil, rocks, and minerals.
Figure 1. Ion chromatograms of rainwater samples. The samples in a and c were from storms that came from the west and over land. The sample in b was from a storm that came in from the east and over the ocean.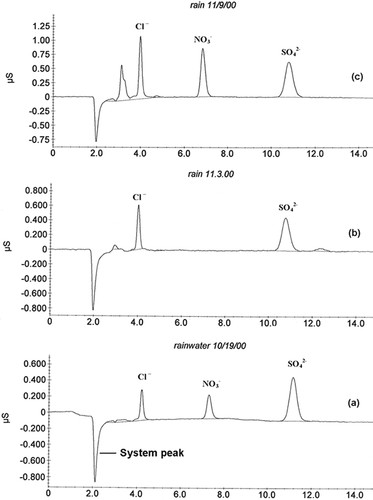
In earlier offerings of the course, the students submitted a brief report on the iron leaching experiment. Now, another purpose of this experiment is to give students practice at writing a report in the form of a chemistry publication in the peer-reviewed literature, which is the same format used for the final report on the lead project. We go over a handout that describes the format of a scientific paper. Each student writes an individual report, and I provide them with thorough comments designed to guide them in writing an improved report for the final project. The credit for the iron report is nominal when compared with the project report. By instituting a more formal report for the iron experiment and providing thorough feedback, there has been a noticeable improvement in the quality of the final written project reports.
The importance of greenhouse gases in causing global warming is discussed in the class, and this information is supplemented by a laboratory analysis of the infrared absorption spectra of carbon dioxide, a freon (CCl2F2), and methane. Using the computational software Spartan, the students first visualize the different vibrational modes for the three compounds and then calculate the energy of each. After a discussion of the selection rules for allowed vibrational excitations, which involves a change in the molecular dipole during the vibration, the students are able to determine the allowed vibrations for each compound. Each group then measures the infrared absorption spectra for the three compounds and compares the actual energies of the allowed vibrations to the calculated values. The calculated values are not exact, but they are close enough to ensure the proper identification of the vibrational absorptions. Finally, we overlay the spectra of the three compounds to show the complementary nature of the absorptions, thereby appreciating how these gases, in the aggregate, contribute more to global warming than any one of the gases individually.
The final experiment involves using gas chromatography–mass spectrometry to analyze hydrocarbons in air that come from automobile exhaust (methyl benzenes) and pine trees (terpene hydrocarbons). Using a special injection system designed for use in the flavor and fragrance industry, the students are able to identify the major terpenes that make up the aroma of pine needles. Then, with a battery-powered air-sampling device that uses compound absorption onto Tenax traps (Kroupa et al., 2004), the students take air samples next to a busy road and in a pine grove. An example of the results that are obtained is shown in Figure 2. The sample taken on the road (Figure 2a) has much higher concentrations of anthropogenic methyl benzenes (compounds 1-6) relative to natural terpenes (compounds A–C). The sample taken in the pine grove (Figure 2b) has much higher concentrations of the terpenes relative to the methyl benzenes.
After completion of these experiments, the plants have been growing for about 6 to 7 wk, and it is time for harvesting. Plants are cut off at the soil line and dried for several nights in an oven. The building smells like steamed vegetables during the initial drying steps so that everyone knows that it is harvest time in general chemistry. We have never analyzed the roots, assuming that it is unlikely that we would be able to wash the roots well enough to remove all the lead that might adhere to the surface in the contaminated samples. Also, we have never segregated plant samples into different parts (e.g., leaves and stems) as a way of keeping the number of samples to be analyzed to a manageable level.
Plant samples (up to 1 g if available) are digested in beakers on a hot-plate using concentrated nitric acid (10 ml). The students are instructed to carefully reduce the volume to ∼2 ml. At this point, the nitric acid seems a bit cloudy, but very little plant material remains in the beakers. Samples are then diluted to 50 ml with distilled water and stored in plastic bottles until analyzed by inductively coupled plasma-atomic emission spectroscopy (ICP-AES). Before analysis on the ICP, ∼10–15 ml of each sample is filtered through a 0.45-μm filter. Our ICP has an array detector so the groups are provided with printouts of their data and asked to verify that the relative concentrations reported by the ICP are in agreement with a visual inspection of the spectra. Figure 3 shows a comparison of the output from the ICP-AES for plants grown in contaminated and uncontaminated soil. In this example, the soil had been contaminated with leaded paint dust and had lower levels of lead than when we use lead acetate. The top two traces are from plants grown in contaminated soil, the bottom two traces are from plants grown in uncontaminated soil. It is obvious that the plants grown in the contaminated soil had higher concentrations of lead. The background emission apparent in these spectra as well as the obvious emission peak from an impurity that occurs to the right of the lead peak indicates the care that must be taken to properly correct for background emission, especially when the lead levels are relatively low.
Meeting with the entire laboratory section, we then verify that each group does a proper calculation to get the actual amount of lead in the dry weight of the plant. This is necessary because some of the groups may not have had enough dry weight of plant material to get a full gram of sample, and all groups need to remember that 1 g of plant sample was diluted by a factor of 50 during the workup. The groups then report their results to the rest of the laboratory section, and we tabulate all of them on the blackboard. As a group, we then analyze and discuss the composite data to look for trends related to the two questions that were posed at the start of the semester. The students are instructed to focus the discussion in the final written report on their own samples and data but to compare the conclusions from their group's data with those of the entire laboratory section to see whether the same conclusions are generally reached. Each student submits an individually written report, although they are encouraged to discuss their conclusions with the group and to verify with each other the specific experimental aspects that will be described.
Two interesting results have surfaced every year that I have done the lead project. The first result is that almost all of the groups find that the plants grown in the contaminated soil have higher lead levels. The plants growing in contaminated soil tend to have ∼10–100 ppm lead on a dry weight basis, whereas plants grown in uncontaminated soil have 5 ppm or less. These values are in reasonable agreement with measurements reported in the literature (Zantopoulos et al., 1992; Weatherford et al., 1997; Pichtel et al., 2000; MacFarlane and Burchett, 2002; Piechalak et al., 2002; Olivares, 2003). For example, in 1 yr, we had a total of 29 comparisons between contaminated and uncontaminated soil, and in 26 cases, the plants grown in the contaminated soil had higher levels of lead. The students are generally perplexed when deciding what to do with the small number of samples that do not agree with the original hypothesis. Often, the students with the anomalous data conclude that they must have mislabeled or contaminated the control samples in some way, although they have no basis on which to make such a claim. Many students simply want to ignore the outliers because they are contrary to our hypothesis. This provides a chance for us to discuss the ethical implications of ignoring data that do not show the expected trend when there is no justifiable reason to exclude it. Given recent stories that have been in the news concerning certain pharmaceuticals, I ask them whether they would feel comfortable with a company ignoring data that indicated that three in 29 people had an adverse reaction to a drug. We also discuss how the overwhelming amount of data do support our hypothesis, and stating that plants growing in lead-contaminated soil are likely to take up more lead is a reasonable conclusion based on our data.
Figure 2. Gas chromatograms of air samples taken by the side of a busy road (a) and in a pine grove (b). Compounds 1 (toluene), 2-4 (C2-benzenes), and 5-6 (C3-benzenes) originate primarily from automobile exhaust. Compounds A (α-pinene), B (camphene), and C (β-pinene) originate from emission from pine trees.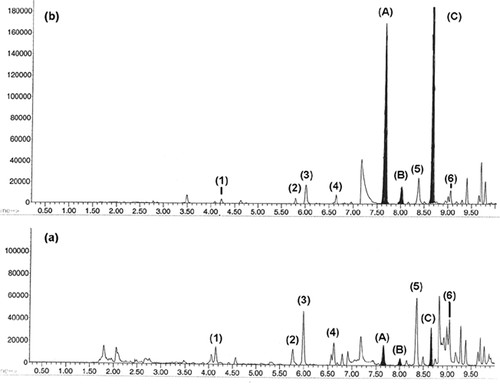
The second interesting result is that the data for lead uptake as a function of acidity, when examined over several groups or the entire class, show no consistent trend. For every group that has data showing higher lead levels at higher acidity, there is a group with the opposite trend. In any given year, several groups that studied three different pH values find that the lead is highest for the middle value. The students are quite uncertain what to make of these data, and it provides a chance to discuss the subtlety of the trend we are investigating. We consider that different groups grew different plants and that the effect of acidity on lead uptake may be species dependent. We also consider that we did very few samples over a very short time. Reflecting back on the small amount of acid that was needed to prepare solutions with a pH of 4 and 5, the students appreciate that a thorough examination of the effect of acidity on lead uptake would require a much more detailed and extensive experiment and that more thorough control of variables would have to be instituted. Reflecting back on the effect that the schist had on the pH, the students appreciate that the soil may have similarly influenced the pH through its buffering capacity. The data obtained for the two questions posed in the project provide an excellent appreciation for the way in which different degrees of uncertainty are inherent in any scientific investigations.
Figure 3. Printout of the response (counts per second; cps) on the subarray of the inductively coupled plasma that contains the lead 220.346-nm emission line. The top two traces are from plant samples that were grown in soil contaminated with lead. The bottom two traces are from plant samples grown in uncontaminated soil.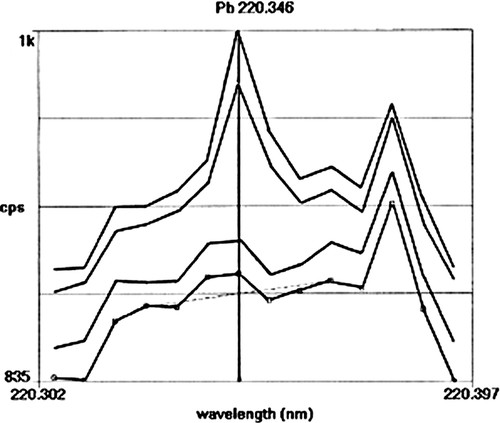
LABORATORY OUTCOMES
Undertaking the small-group, semester-long laboratory project coupled with other, shorter experiments allows me to meet a broad range of learning outcomes. Students gain experience with fundamental laboratory skills such as weighing, preparing solutions, performing measurements, and constructing standard curves. They also get experience using standard pieces of equipment such as a pH meter, spectrophotometer, and volumetric glassware. Unlike most general chemistry laboratories, the students are not provided with any solutions but have to make up everything on their own. Informal surveys of upper-level chemistry and biochemistry majors indicate that students who have taken the course feel well prepared with respect to fundamental laboratory skills expected in upper-level chemistry and biochemistry courses.
The project and group work provides students with the opportunity to develop many of the other learning outcomes described above, although I admit that I neither measure many of these skills at the start of the semester nor have I formalized a mechanism to measure student growth in many of these areas. Even without a formalized assessment of the degree to which the students learn to think critically, improve their communication skills, develop self-respect and respect for others, change or solidify their views on ethical scientific practices, or learn better skills for collaborating within a team project, I believe it is better to provide a laboratory experience that offers the opportunity for growth in these areas rather than a laboratory experience that does not.
Students in the course must assume responsibility for their project and make a number of decisions about how to set up and carry out the experiment. They have to adhere to a watering schedule and often encounter unanticipated problems that must be addressed during the course of the semester. These problems have included, among others, occasional problems in accessing the greenhouse at off hours, a bed of plants that is not growing, and a leak in the greenhouse roof during an exceptionally strong storm that drenched many of the plants.
Students get experience working as part of a team in which they must depend on and communicate with each other. There is an opportunity for students to assume a leadership role within their group and to develop self-respect and respect for others. Interpretation of the data requires critical thought and analysis. The students also get experience writing information in the form of a scientific publication. I often find that the geology majors have extended sections in their introduction on soil and mineral science and that biology majors often write about details of plant growth and nutrient uptake. The nature of the project allows students to tailor aspects of the final report to their particular interests. This particular project readily links aspects of chemistry, biology, geology, and environmental studies such that students better appreciate the connections among these disciplines. Quantitative skills are needed to complete the experimental aspects of the project and to analyze and present the data, which in a modest way fit with the growing emphasis on bridging biology topics to skills in mathematics.
A few years ago I instituted a peer and self-evaluation process as a summative assessment at the end of the term ( http://www.bates.edu/Prebuilt/212-peerself-05.pdf). These evaluations indicated that most of the groups functioned well and that the majority of the students were full and equal contributors to the project. Because these evaluations indicated that some groups experienced problems in which an individual or two failed to contribute equitably in the execution of the project, I now use the peer and self-evaluation as a formative process at the halfway point of the semester to identify and intervene with students who are not full participants. I meet with these individuals to explain my expectations and describe the improvements I hope to see in the final peer evaluation. In almost every case, this meeting had the desired outcome, in part because the students usually admit in their self-evaluation that they have not contributed as much to the project as they should. I also talk to the diligent students to express my appreciation for their efforts and to inform them that I am aware of the disparity in contributions to the project and that I anticipate that the situation will change. In the aggregate, the comments in the peer and self-evaluations indicate that the students appreciate the sense of shared enterprise that characterizes the project. The peer and self-evaluations are used as an aid in assigning the participation portion of the laboratory credit.
I have found that the nature of the activities needed to complete the project coupled with the peer and self-evaluations provide me with a much fuller sense of each student's strengths and weaknesses. By having the students work in groups and giving them the opportunity to make decisions and design the experiment, I know which students exhibit leadership qualities and which offer thoughtful suggestions about how to execute the project. I have a better sense of each student's work ethic, and I am able to assess each student's ability to work as part of a team. As the groups meet to examine and interpret their final measurements, I gain an understanding of their critical thinking skills. I have found that I can write far more informative and useful letters of reference than I could with a more traditional laboratory format.
STUDENT RESPONSE
Student response to the course is gathered through a formal evaluation of teaching administered by the college at the end of each semester, a formal laboratory evaluation, and informal feedback I receive from students. This feedback strongly endorses the value of having students undertake such a project at the introductory level. Students express considerable satisfaction with the laboratory experience as a whole. About 90% favor the semester-long project over a format of weekly or a few multiweek experiments. The same percentage feels that they did not miss out on other important laboratory experiences by undertaking the project. They expressly like the independent nature of the project and that they are allowed to make many of their own decisions in its implementation. They like having access to the greenhouse, which is a restricted area. Some have expressed appreciation that they are doing “real science.” A number have said over the years that it was the first time they ever felt like they were actually doing science rather than just learning about science. Students also like the connection among disciplines. Geology majors have said how they liked that they could share some of their knowledge of soils and minerals with other students in their groups. Many biology majors appreciate that we are growing plants and that they have insights to share with group members. Environmental studies majors express appreciation for the development of a chemistry course that meets their specific needs and interests. Indeed, two environmental studies majors have gone on to conduct senior thesis projects that are offshoots of the lead project. One senior monitored the lead levels in urban soil at sites being used as community gardens. The other senior examined the potential of using spinach with added chelating agents as a means of phytoremediation for lead-contaminated sites. Some of the strongest positive responses to the course have come from students who are taking the course to fulfill the college's general education science requirement and therefore are not majoring in a science discipline. They appreciate the broader skill set emphasized in the course and especially like that not all of the graded assignments for the classroom part of the course are quantitative exams but include essays as well. Over the years, several of these students who only needed the first semester of the sequence to complete their general education science requirement went on to take the second semester. Students also value the group activities with almost all of them expressing that they are “quite satisfied” or “satisfied” with the functioning of their laboratory and classroom groups.
When I first developed the lead project, I anticipated that I would use it for a few years and then switch to another semester-long project. Instead, I have found that the project remains continually fresh for me and the students. I never know what to expect until we finally write the measured lead levels on the board in the last laboratory period. The variety of plants we can grow, coupled with the ability to vary aspects of the acidity and lead contamination of the soil, has created an environment in which the students undertake a project that legitimately demonstrates the scientific process and what is involved in undertaking scientific research. The project also has an excellent set of interdisciplinary connections that further enhances its utility in an introductory course. Fortunately, I am in a department that embraces the value of inquiry so that the skills started in my course at the introductory level are further developed throughout our curriculum.
FOOTNOTES
Monitoring Editor: Marshall Sundberg
ACKNOWLEDGMENTS
National Science Foundation Grant DUE-9950314 enabled us to purchase the ion chromatograph and gas chromatograph–mass spectrometer.



