From Organelle to Protein Gel: A 6-Wk Laboratory Project on Flagellar Proteins
Abstract
Research suggests that undergraduate students learn more from lab experiences that involve longer-term projects. We have developed a one-semester laboratory sequence aimed at sophomore-level undergraduates. In designing this curriculum, we focused on several educational objectives: 1) giving students a feel for the scientific research process, 2) introducing them to commonly used lab techniques, and 3) building skills in both data analysis and scientific writing. Over the course of the semester, students carry out two project-based lab experiences and write two substantial lab reports modeled on primary literature. Student assessment data indicate that this lab curriculum achieved these objectives. This article describes the first of these projects, which uses the biflagellate alga Chlamydomonas reinhardtii to introduce students to the study of flagellar motility, protein synthesis, microtubule polymerization, organelle assembly, and protein isolation and characterization.
INTRODUCTION
Several pivotal studies over the past decade have suggested that new approaches are necessary to improve undergraduate science education (Howard Hughes Medical Institute, 1996; National Science Foundation, 1996; National Research Council, 1997, 2003). A common criticism of many traditional undergraduate laboratory experiences is that each lab session is an isolated activity unrelated to the following week's lab; such lab activities leave students with an inaccurate view of how scientific research is conducted (Holt et al., 1969; National Science Teachers Association, 2001). Although students often are required to write lab reports based on one lab session, a biologist would never publish an article based on a single experiment that relied on one technique. Indeed, several techniques used in series are needed to allow the investigator to draw conclusions. Thus, the practice of having students carry out an isolated experiment once a week, and then write weekly lab reports based on these experiences, is unfortunate in several respects. Students in such labs are led to view scientific research as consisting of isolated procedures that take only a few hours and that produce enough data to draw conclusions, rather than viewing research as a multistep process. A growing awareness of problems such as this has led to the development of project-based laboratory courses. Although there are many published descriptions of project-based laboratories, including several lab manuals, in which students carry out a semester-long project, or projects that require more than one lab session per week (Karcher, 1995; Stukus and Lennox, 2001; DiBartolomeis and Mone, 2003), there are few that involve shorter projects aimed specifically at lower-level undergraduates.
We have developed a lab experience for a one-semester, sophomore-level course in cell and molecular biology that attempts to deal with several of these issues. In developing this lab sequence, we had several objectives. First, we wanted the students to get a sense of the continuity of lab research; to realize that a single “experiment” may consist of several techniques and may take days (or weeks) to accomplish. Second, we hoped to highlight the connections between a living organism and the molecules of which it is composed. Third, we wanted to expose students to a variety of common lab techniques. Last, but certainly not least, we sought to improve the students' ability both to write scientific prose and to think critically and analyze data.
The student population taking this course is predominantly first-semester sophomores, with limited experience in writing formal lab reports. Therefore, one of our strategies was to give the students several opportunities to develop scientific writing skills. A single, semester-long project culminating in a single, long lab report would not have achieved this goal. In addition, because this is a course in both cell and molecular biology, we hoped to introduce students to a range of experimental techniques encompassing both fields. Therefore we developed a lab curriculum that splits the semester roughly in half, so that students carry out two major projects and write two substantial lab reports. After 1–2 wk in which students are exposed to some fundamental techniques (e.g., microscopy, sterile technique, or the use of micropipettors), the students carry out a project that takes 3–4 wk. Each half of the semester thus consists of an integrated series of lab experiences and culminates with a lab report written in the format of a scientific paper.
The first of these two lab sequences takes students from an examination of cellular behavior (flagellar motility) in a live organism to isolation and partial characterization of the proteins involved in flagellar motility. This experimental series exposes students to several important techniques, including phase-contrast microscopy, cell fractionation, colorimetric protein assay, and SDS-PAGE; it also requires students to formulate and test a formal hypothesis. Overall, these experiments are designed to help students begin to answer the question, “How, and of what components, are flagella built?” Thus, the flagellum serves as a model organelle with which we convey to students both the overall complexity of eukaryotic cell structures and some notion of how such complex organelles can be assembled. In this report we present this experimental sequence, which uses the model organism Chlamydomonas reinhardtii. C. reinhardtii, commonly referred to simply as Chlamydomonas, is a biflagellate, unicellular, green alga used for a variety of studies, especially in the areas of flagellar motility and photosynthesis (for more information, or to obtain a variety of wild-type and mutant strains, see The Chlamydomonas Genetics Center; Harris, 2005). Among the useful attributes of this organism are that it is easily grown at room temperature in simple inorganic medium; it has a haploid genome with well-characterized genetics, including numerous mutant strains; and it is both photosynthetic and motile. These characteristics make it well suited for use in undergraduate teaching laboratories and research, and a Web site supporting such use has been developed by Mike Adams: The Chlamydomonas Teaching Center (Adams, 2005).
The flagellar lab sequence, including both the introductory labs and the actual project, is accomplished over 6 wk in a standard, once-a-week lab session of 3 h (Table 1). Of this, 5 wk entail actual bench work and 1 wk is reserved for meeting with students, either individually or in groups, depending on instructor preference. This makes it suitable for use in a large multisection lab course; we routinely run four lab sections for our course, with students working in groups of three to four. One strategy we have used to maximize the efficient use of both student and faculty time is to schedule a prelab for our course. In the required prelab, students in all lab sections meet together once a week for 50 min. During that period, the lab instructor may give background information for the coming week's lab, reinforce basic skills in science writing, or go over any calculations or data analysis that may need to be done between one experiment and the next. For example, prelab time is used for teaching students how to create and use standard curves for determination of protein concentration and molecular weight. This lab sequence gives students a feel for the scientific research process, without actually having them develop and carry out independent projects. Although research is an ideal way to engage students in the practice of science, it is not always feasible. In this case, the large number of students taking this course (65–80 each fall), combined with their limited lab experience as first-semester sophomores, and the absence of teaching assistants, made it impractical to have students carry out complex, independent research projects. The flagellar lab sequence described here attempts to strike a balance between directed experimental activity and independent research.
| Date | Experiment | Report due |
|---|---|---|
| Week 1 | Microscopy | Week 2 |
| Week 2 | Cell Motility | Week 3 |
| Week 3 | Flagellar Regeneration | Week 8 |
| Week 4 | Isolation of Flagellar Proteins | Week 8 |
| Week 5 | Separation of Flagellar Proteins by SDS-PAGE | Week 8 |
| Week 6 | LONG WEEKEND—No labs all week, BUT … on Thursday, you must meet with lab instructor to discuss preliminary results (tables/figures); draft of Materials & Methods due.a | |
| Week 7 | NO LABS—WORK ON LAB REPORT. We WILL have prelab and groups must meet with lab instructors; graphs and tables due; draft of Introduction due. |
PROJECT BACKGROUND
A hallmark of eukaryotic cells is the presence of complex organelles that must be built up from component parts. Flagellar regeneration is an easily observed example of such organelle assembly and serves to illustrate the importance (and complexity) of such processes. Chlamydomonas has been a key model system for developing our understanding of flagellar assembly, including the process of intraflagellar transport (IFT). IFT is required for flagellar assembly and maintenance, and much work in the past decade has shown that correct flagellar assembly is essential not only for motile cilia and flagella but also for the nonmotile primary cilia found on nearly all metazoan cells. Primary cilia are involved in sensory transduction, and homologues of both IFT and basal body genes are associated with a variety of human diseases such as polycystic kidney disease, primary ciliary dyskinesia, retinitis pigmentosa, and Bardet–Beidl syndrome (for reviews, see Pazour and Rosenbaum, 2002, Snell et al., 2004).
Eukaryotic flagella are composed of numerous polypeptides (for review, see Luck, 1984) in a complex ultrastructural arrangement; however, the major structural framework of the flagellum consists of the 9 + 2 arrangement of microtubules known as the axoneme. Flagellar beating involves sliding between adjacent outer doublet microtubules; sliding is driven by the dynein arms, large motor protein complexes attached to the outer doublets. Microtubules are assembled by the polymerization of protein heterodimers composed of α- and β-tubulin; tubulin dimers add on to the distal end of a growing flagellum (Rosenbaum et al., 1969). One feature of Chlamydomonas that makes it particularly useful for studies on flagellar motility is the ease with which cells can be induced to drop their flagella, either by a sudden drop in pH (pH shock) or by exposure to any of several drugs, including the anesthetic dibucaine. After deflagellation, cell bodies can be readily separated from flagella by centrifugation. When resuspended in fresh medium, cell bodies regenerate their flagella in ∼90 min, in a process that involves both protein synthesis and polymerization of tubulin. Flagellar regeneration can be completely and reversibly blocked by the drug colchicine (Rosenbaum et al., 1969), which binds to tubulin dimers and prevents microtubule polymerization. In the absence of colchicine, regeneration begins rapidly, initially using a pre-existing cytoplasmic pool of tubulin, and then using newly synthesized polypeptides to achieve full length. The pool of premade tubulin allows for partial regeneration (flagella typically reach 1/3–2/3 full length) when translation is blocked by cycloheximide. Although deflagellation up-regulates the synthesis of flagellar mRNAs (Silflow and Rosenbaum, 1981), blocking transcription with actinomycin D has little or no apparent effect on flagellar regeneration, because cells in medium containing this drug can regenerate full-length flagella (Vandewalle and Heyes, 1993), indicating the presence of a pool of tubulin mRNA in addition to the existing pool of protein. Regeneration in actinomycin may occur more slowly than in control cells (Figure 1), although this is not always the case. It has been suggested that deflagellation may change the stability or activity of tubulin mRNA, thus allowing regeneration in the absence of new mRNA synthesis (Baker et al., 1984; Vandewalle and Heyes, 1993). In addition to drugs, microtubule polymerization and flagellar regeneration are inhibited by cold (Behnke and Forer, 1967).
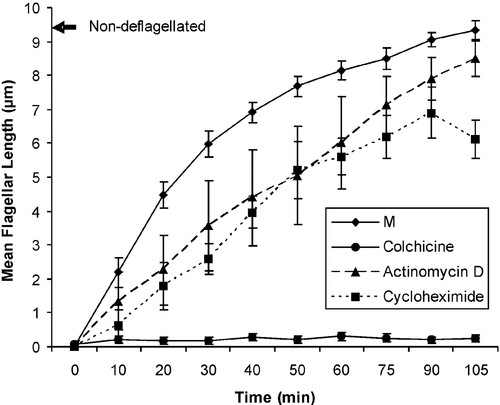
Figure 1. Flagellar regeneration. Mean flagellar length (micrometers) ± SEM versus time after deflagellation. C. reinhardtii were deflagellated at time 0 and then allowed to regenerate in minimal medium (M), or M containing 3 mg/ml colchicine, 10 μg/ml cycloheximide, or 50 μg/ml actinomycin D. Pooled data from an entire class (all groups in each of four lab sections, n = 17) were used for curves representing control (M) and colchicine conditions; pooled data from the subset of groups using each particular experimental condition were used for curves representing cycloheximide and actinomycin D (n = 5). Mean flagellar length of nondeflagellated controls measured at 0 and 105 min (9.3 μm) is indicated by the arrow.
Chlamydomonas flagella have been characterized by both one- and two-dimensional electrophoresis and are known to contain >250 polypeptides (for review, see Luck, 1984). One-dimensional SDS-PAGE and Coomassie blue staining allows the visualization of only a few of these polypeptides; nevertheless, it is still apparent that this organelle contains numerous proteins, which are present in differing quantities. Tubulin (55 kDa), the most abundant protein, occurs as a very intensely stained band. Heavy chains (300–500 kDa) of the dynein motor complexes, whereas considerably less abundant than tubulin, are still visible near the top of Coomassie-stained gels. Numerous other protein bands are visible as well, although identification of most of these would require additional information.
EXPERIMENTAL OUTLINE
During the introductory phase of this project, students spend 2 wk in preparatory activities. The first week of the semester is devoted to a standard microscopy lab, including calibration and use of the ocular micrometer; this lab serves to familiarize students with the phase-contrast microscopes they will be using. Although this laboratory project can be done using bright-field microscopy, phase contrast is preferable, because flagella are much easier to view with phase-contrast optics. During the second week, the students continue to use phase-contrast microscopy while studying cell motility. This lab session introduces the students to microfilament- and microtubule-based motility in a variety of living cells, including C. reinhardtii (see Supplemental Material 1; instructor prep sheets for each lab protocol can be found in Supplemental Material 2). Flagellar motility in wild-type C. reinhardtii, as well as several mutant strains with motility defects, is analyzed using both microscopy and a simple phototaxis assay (Harris, 1989). All organisms for this laboratory are readily available from Carolina Biological Supply (Burlington, NC) or Ward's Natural Science (Rochester, NY), apart from the C. reinhardtii mutant strains, which are available from The Chlamydomonas Genetics Center (Harris, 2005). For the motility lab, students must turn in a short lab report consisting of a brief Introduction followed by a combined Results and Discussion section. The grading rubric used by the instructors (Supplemental Material 2) is made available to the students. This assignment is used to emphasize basic skills in scientific writing, including proper use of genus and species names, use of past tense, and correct citation format; in addition, this assignment allows early intervention for students needing referrals to the college writing center.
The major project begins with an experiment on flagellar regeneration (Supplemental Material 1). This experiment has been adapted from one published by Bregman (1990) and is based on work from Joel Rosenbaum's lab (Rosenbaum et al., 1969; Lefebvre and Rosenbaum 1986). Bregman's student lab has been used in modified form by a number of others (Adams, 2005); our major innovation is in integrating it with other experiments that use flagella. A sample of C. reinhardtii is deflagellated by pH shock and then allowed to recover in the presence or absence of colchicine, which inhibits microtubule polymerization, and thereby prevents flagellar regeneration. Working in small groups, students fix cells by using a nontoxic iodine solution, and then they use phase-contrast microscopy to measure flagellar length in 10 cells from each experimental population: nondeflagellated cells, cells regenerating both in control medium and in medium with colchicine, and cells regenerating under one other experimental condition of the group's choice. The need for positive and negative controls in experimental design is discussed, and students predict the outcome of the control conditions for regeneration. For their experimental condition, choices include exposing regenerating cells to drugs affecting transcription or translation (actinomycin D or cycloheximide, respectively), testing to see whether the effect of colchicine on regeneration is reversible, and testing the effect of cold. For the experimental condition of their choice, student groups construct a formal hypothesis that must be approved by the instructor. Time during prelab is devoted to a discussion of what constitutes a valid scientific hypothesis and how to formulate such a hypothesis, and then students work with their lab partners to clearly state their hypothesis; immediate feedback from lab instructors is used as an opportunity to help students develop their problem-solving skills. Examples of such student hypotheses can be seen in Table 2. As evidence that this approach represents an improvement in ability, and not merely that students could already formulate a valid hypothesis, we have included three typical examples of students' first attempts at drafting a hypothesis compared with their final hypothesis (Table 3); for one of these examples, we have included a second draft as well.
| Student | Hypothesis |
|---|---|
| 1 | “In the case of cycloheximide, it was hypothesized that if flagellar regeneration requires protein translation, then deflagellated Chlamydomonas reinhardtii will not regenerate flagella completely. Flagella will only be synthesized with cytoplasmic tubulin dimers, since no new tubulin can be produced.” |
| 2 | “Our hypothesis is if flagellar regeneration is dependent on the translation of mRNA to protein, then cells exposed to cycloheximide will not regenerate their flagella.” |
| 3 | “The hypotheses was that if the regeneration of flagella on C. reinhardtii is related to the transcription of mRNA than the addition of actinomycin D will prohibit μt polymerization not allowing regeneration o the flagella.” |
| 4 | “We hypothesized that, if protein synthesis is needed for the growth of microtubules, which make up cilia and flagella, then the addition of the drug cycloheximide, that causes inhibition of protein synthesis, will not allow flagellar regeneration to occur.” |
| 5 | “In this lab it was hypothesized that if temperature is related to flagellar regeneration, then deflagellated Chlamydomonas reinhardtii exposed to a temperature of 4°C will have little to no flagellar regeneration. This was based on the idea that proteins and enzymes have optimum temperatures at which they work best, and such a cold temperature would interfere with their functions in microtubule polymerization in the flagella.” |
| Draft | Hypothesis |
|---|---|
| Original A | “If, the drug colchicine inhibits the microtubule polymerization then, by discontinuing colchicine administration will reverse these effects which will then reestablish the microtubule polymerization translation sequence for protein that make flagella.” |
| Final A | “If the effects of colchicine are reversible then, by washing away the drug should allow microtubule polymerization to be reestablished.” |
| Original B | “If cold temperature is related to flagellar growth then a decrease in temperature will decrease the growth of flagella.” |
| Final B | “If flagellar growth is dependent on temperature then a decrease in temperature will decrease the growth of flagella.” |
| Original C | “If actinomycin D is applied to the flagella of Chlamydomonas reinhardtii then their growth will be inhibited.” |
| Second C | “If actinomycin D inhibits transcription during protein synthesis then the synthesis of new alpha and beta tubulin subunits will be smaller than those not exposed to actinomycin D.” |
| Final C | “If transcription is necessary for flagellar regrowth then the presence of actinomycin D should prevent flagellar regeneration.” |
Student data for these experiments are pooled, so each student has data from all lab groups (generally a total of 18–20 groups); this pooling allows for a statistically better sample size (even the individual experimental conditions are generally repeated by several groups) and for students to see the results of experimental conditions other than their own. Students calculate average flagellar lengths, which allows for review of the concept of significant figures, and plot mean flagellar length versus time. For a sample graph of student data, see Figure 1. The data for positive and negative controls (regeneration in control medium and regeneration in the presence of colchicine) have much smaller SEs, because these controls represent data from all groups in the class (n = 17 groups), whereas the individual experimental conditions represent data from only five groups. We do not actually require students to calculate SEs for these data, because most of our students have not yet taken statistics and are unfamiliar with statistical analysis; however, we have included error bars in Figure 1 to illustrate the range of student data. The n for these measurements differs between conditions for several reasons. First, although all groups carry out regeneration under control conditions (M, colchicine, and nondeflagellated), the experimental conditions are carried out only by certain groups, so the total number of flagella counted is different for those conditions. Second, there is not uncommonly a situation in which one or more individual students makes an error (e.g., taking samples from a tube labeled “colchicine” rather than a tube labeled “cycloheximide”). Although some errors are caught during lab time, others inevitably are not discovered until all the groups' data are gathered. At that point, we review the data and sometimes tell the students to not use certain data, if it is clearly incorrect. For example, recently we had a student who seemed to have flagella with an average length of 6 μm at time 0. Because this is immediately after deflagellation, virtually the entire cell population completely lacks flagella, thus this number was clearly wrong. On questioning this individual, we discovered he had been searching the slide for the few remaining cells that had not dropped their flagella (a very tedious endeavor) so he had something to measure, rather than simply looking at the first 10 cells he saw. After explaining to the class that one does not ordinarily “decide” whether or not to use data, we told them that this was an exception (and why) and told them to omit his data from their final calculations. One way in which the lab report differs from a research paper is that regeneration data are presented both in table and graph formats. This redundancy serves two pedagogical purposes. First, students must learn to use correctly both forms of data presentation; and second, this approach allows for discussion of how different sorts of data can best be presented.
During the second week, students work in groups to isolate flagella from C. reinhardtii by using a modification of the procedure of Witman (1986) (Supplemental Material 1). Washed cells are deflagellated with dibucaine, and flagella are purified by differential centrifugation. Protein assays are run on samples of isolated flagella as well as samples of whole cells and of cell bodies lacking flagella. The protein concentrations are used to determine sample volumes to be loaded onto gels. Equal loads of total protein (in micrograms) for each sample are run, allowing students to judge qualitatively what fraction of the total protein in cell bodies versus flagella is represented by polypeptides of the same molecular weight (e.g., tubulins or dyneins). During the third week, students separate proteins in these samples by SDS-PAGE on 4–15% gradient mini-gels (Bio-Rad, Hercules, CA; Supplemental Material 1). Although nongradient polyacrylamide gels can be used, the wide range of molecular weights found in flagellar polypeptides (from dynein heavy chains of ∼500 kDa to dynein light chains of <10 kDa) is better resolved on gradient gels. After staining and photographing their gels, students determine the molecular weights of selected polypeptides. Because students are encouraged to choose intensely stained bands, most groups will choose the tubulin band (this being the most abundant protein in the flagellar sample), allowing them to tentatively identify the polypeptide after determining its molecular weight. Dynein heavy chains, another major constituent of flagella, are also likely targets for students to identify, based on both relative abundance and high molecular weight. In addition to being a source of quantitative data (i.e., molecular weight), gels are examined for more qualitative information, for example, comparisons of banding patterns between whole cell, cell body, and flagellar samples to determine similarities and differences in protein content. During pre-lab discussions, and while reviewing drafts of lab reports, we encourage students to think about not only why some samples (whole cells and cell bodies) have more similar protein content than others (flagella) but also what it means that some proteins (e.g., tubulin) are present in all samples, particularly in the context of their earlier results on regeneration in the presence of inhibitors of protein synthesis.
After completing the series of experiments, each student must write a lab report in the format of a journal article. Because they have carried out several distinct procedures, students are forced to think about the connections between form and function: protein content, protein synthesis and assembly, and flagellar beating. To assist them, the students have access to a variety of material through the course Web site, including a list of “Do's and Don’ts” based on common errors and the grading rubric used by instructors (Supplemental Material 2). The latter tells students what we are expecting to see in their reports, thereby serving as a guide for them as they write what is usually their first lengthy lab report. After completion of the experimental work, a final laboratory period in this sequence is devoted to meeting with students to discuss graphing and interpretation of data and to look over required preliminary drafts of their Introduction and Materials and Methods. Additional information is presented to students in the prelab, where discussions focus on interrelated concepts from both lab and lecture. In the latter, for example, we will have recently studied how proteins can function both as structures and as machines, and how the cytoskeleton serves as an example of both types of protein function. In particular, we emphasize that the lab series takes them from a functional organelle through its assembly from tubulin and other proteins to a preliminary analysis of the proteins that make up the organelle. Our students are required to purchase a text on scientific writing (we have used several over the years) while taking general biology, thus they already have this resource available when taking our course. The due date for the final report is 2–3 wk after completion of the experimental work (Table 1), so students have sufficient time to devote to data analysis and writing.
There is evidence in the student lab reports that they begin to see the connections between isolated proteins and cell structure and function. For example, in their Introduction, students tend to write about tubulin being a dimer, that it assembles into microtubules, and that the microtubules are arranged in the 9 + 2 pattern in the flagellum. They state that microtubules are also in the cytoplasm and are used to make the spindle apparatus for chromosome movement in mitosis. They also state the action of the drugs and their effects on microtubule assembly, or transcription and translation of tubulin. In the discussion, students usually figure out how the assembly of microtubules can be understood in relation to the known effects of these drugs. For example, they conclude that the cells seem to have a pre-existing pool of tubulin that allows partial regeneration when translation is inhibited. This interpretation is then reinforced by the SDS-PAGE data that show that the cell bodies contain tubulin. Students also can see from the gel that tubulin is more abundant in the flagella than in the cell bodies. Therefore, SDS-PAGE reinforces the concept that the cell must have to make more tubulin for flagella to reach full length. Some students tie in the concept that the tubulin in the cell bodies also can be used for the formation of the mitotic spindle.
ROLE OF THE LAB SEQUENCE IN THE CURRICULUM
The Cell and Molecular Biology course for which this lab series was developed plays a key role in our departmental curriculum. The course is a graduation requirement for all biology majors and minors, and biochemistry majors, as well as being a prerequisite for many upper-level biology classes. Although it is not mandatory that students take this course in their sophomore year, this is typically when it is taken. Thus, for most students this course is the first majors' course taken after a two-semester general biology sequence. In the General Biology course, students are assigned several short lab write-ups and a poster presentation based on a group research project. The lab report and experimental sequence described in this article serve both to reiterate previously learned basic skills and to expand upon the freshman experience in terms of techniques and equipment used as well as data analysis and scientific writing.
Sophomore students have not yet learned the fundamental fact of lab life that knowing ahead of time what you are going to do means you can do it more efficiently (and often more correctly). Thus, another general skill we emphasize is advance preparation for lab work. One way in which we encourage students to read the lab thoroughly in advance is by giving brief quizzes at the start of each week's lab. The lab quizzes generally consist of two or three short questions designed simply to determine whether the student read the lab. These quizzes make up 10% of the final lab grade, and the lab grade itself constitutes 25% of the overall course grade. A second, and more important, strategy for improving student preparation involves the use of flow charts. For each week's lab, students must create a flow chart of the experimental procedure. The flow charts are required not merely to ensure that students have looked at the lab ahead of time but also to teach them how to create and use flow charts as a tool to improve lab productivity. Because this is a skill development process, and at the start of the semester most students have no idea how to make a useful flow chart, these are not graded but are checked by the instructor, who makes corrections and suggestions. In addition, early in the semester, flow charts are modeled by the lab instructor. Although we do not give a numerical grade for individual flow charts, 10% of the students' final grade is based on their simply having done all of them. The flow charts routinely show dramatic improvement over the course of the semester, going from being either too long or too short to being a useful tool for the student.
After several years of teaching this lab project, we realized that one of the biggest impediments to student success was time allotted to writing the report. No matter how often we told students to start writing early, inevitably a majority of students postponed starting this report until the last minute. To overcome this problem, we recently began requiring students to turn in a preliminary draft of their Materials and Methods 1 wk after completion of experimental work and then a draft of their Introduction and figures the following week (Table 1). We also scheduled a week of lab time after the bench work, which is devoted to meeting with students, either individually or in small groups, to review the draft of their Materials and Methods. We generally meet a second time with students to look over their draft Introduction and figures. In our case, this week usually corresponds to the week of the Columbus Day holiday, when the college is closed for 2 d, and labs are canceled for the week, so we have made ourselves available during lab period times when students are back on campus. The requirement for these drafts has proven beneficial on several counts: the students can no longer postpone the entire report till the last minute, and the instructors can catch many of the errors in the draft text and figures. The instructor feedback allows students to rework their report before handing in the final copy, and thereby increase their final grade by incorporating corrections and suggestions. Although the actual lab reports must be written individually, students are encouraged to discuss data and interpretations with their lab partners.
As mentioned in the Introduction, the lab sequence described here is the first of two sequences carried out during the semester-long course. The second experimental sequence focuses more on traditional molecular biology (e.g., screening a DNA library), and introduces students to a variety of additional techniques. Because students have already been through the process of writing the first lab report, we do not require them to hand in preliminary drafts for the second sequence. However, students may (and do) request faculty to look over early drafts.
ASSESSMENT OF THE LAB EXPERIENCE
To assess the students' perception of how much they had learned from the entire semester's lab experience, we constructed a retrospective evaluation that was administered to junior and senior biology and biochemistry majors and minors (62 surveys were returned for an 86% response rate). This evaluation allowed us to get feedback from students who had taken not only this course but also at least one additional upper-level biology course. Students completing this evaluation had taken an average of 3.5 ± 1.4 additional biology classes with labs since taking the Cell and Molecular Biology course and lab. Results from this survey, shown in Table 4, indicate that students felt the lab experience had improved their technical ability to work in the lab, their ability to analyze and interpret data, and their ability to write formal lab reports. Furthermore, students felt this experience allowed them to better understand and appreciate the scientific research process (Table 4). Students also developed the ability to formulate a valid scientific hypothesis (Tables 2 and 3). Although we were primarily concerned with students' retrospective views of the course, we also administered an equivalent survey to currently enrolled students near the end of the semester. The data for the two sets of students are similar, although students who have taken several additional biology classes have slightly different views on several questions (Table 4). For example, former students tended to rank this lab more highly as “an overall useful experience” and felt more strongly that it had improved their ability to write lab reports. Students currently enrolled in the course, lacking the perspective of hindsight, were more aware of their improved lab technique and ability to make flow charts.
| Question | Former students (n = 62) | Current students (n = 55) |
|---|---|---|
| 1. Over the course of the semester in CMB, do you feel your ability to work effectively in the lab improved in terms of | ||
| a. technique/competence | 4.1 ± 0.7 | 4.4 ± 0.5 |
| b. writing lab reports | 4.0 ± 0.9 | 3.6 ± 0.8 |
| c. understanding of technical/scientific terms | 4.2 ± 0.8 | 3.9 ± 0.6 |
| d. making flow charts | 3.6 ± 1.2 | 4.2 ± 1.1 |
| e. use of standard curves | 3.4 ± 1.0 | 3.9 ± 0.8 |
| 2. Please rate the following aspects of your experience in CMB lab in terms of their usefulness in other courses you have taken. | ||
| a. writing lab reports | 4.0 ± 1.0 | NA |
| b. ability to graph and interpret data | 3.9 ± 1.0 | NA |
| c. understanding of technical/scientific terms | 4.2 ± 0.7 | NA |
| d. lab technique | 4.5 ± 0.7 | NA |
| e. making flow charts | 3.2 ± 1.3 | NA |
| 3. In CMB, the lab experiences were focused around two sets of lab sequences, each of which took several weeks. Did this arrangement | ||
| a. make it easier to understand that scientific research requires sequential linked experiments, as opposed to isolated experiments? | 4.0 ± 0.8 | 4.0 ± 0.9 |
| b. increase your appreciation of the link between experimental work and scientific knowledge (e.g., information presented in textbooks)? | 3.9 ± 0.9 | 3.7 ± 1.1 |
| 4. Were you more likely to concentrate on using better lab technique when your results from one week were needed for the next week's experiment? | 4.2 ± 1.0 | 4.0 ± 1.1 |
| 5. CMB students are presently required to turn in advance drafts of their Introduction, Materials and Methods, graphs, and tables. Do you feel this process allowed you to do a better job than you would have if the only due date was for the final complete report? | 4.4 ± 0.9 | 4.7 ± 0.6 |
| 6. Looking back on the CMB laboratory, was this overall a useful experience? | 4.2 ± 1.3 | 4.0 ± 0.9 |
Although sophomore students generally find the experience of writing a longer lab report and integrating information from several experiments to be quite challenging, it is an experience that serves to increase their ability to write scientific prose, to plot and interpret graphical data, and to integrate several weeks' worth of information (Table 4). Because many of our upper-level majors' courses require similar formal lab reports, students are expected to retain the skills gained in this course and hopefully to refine them over the next two years. Other instructors of upper-level biology courses corroborate our own observations that students in advanced classes have improved writing and analysis skills. Furthermore, we have been told by many upper-level students that they kept this first long lab report and referred to it while writing other lab reports over the next several years. With the benefit of hindsight, junior and senior students generally agree that the sophomore lab was an important experience, as can be seen from sample comments taken from the retrospective survey. These comments were responses to the question, “In hindsight, is there anything you would like to see changed in the way this lab is organized? Do you have specific suggestions for things you feel should be added, removed, or altered?” Of the 31 student responses to this question, 15 were positive, four were negative, and the remainder were not relevant to this article (e.g., regarding the lecture rather than lab, or the room size). Below is a selection of these responses, both positive and negative:
“Lab was especially helpful. The techniques I had learned have been helpful in my upper level lab courses.”
“It would have been helpful to have a better sense of the overall picture and complete goals of longer, multi-week labs.”
“I think it is organized very well! Needing results from the weeks before and writing and analyzing the whole project was an important experience.”
“I think that the lab was organized very well. It helped students organize and structure their work.”
“The instructions for labs sometimes seemed unclear as well as help in performing more difficult tasks i.e., counting for colchicine lab.”
“The labs were a great learning experience in applying lecture information to hands on experience. In labs, with a group of three or four was helpful in incorporating each person's knowledge level of the course with one another.”
“I thoroughly enjoyed the fact we were able to split up the lab report into chunks and hand it in for early assessment. Although the work was cumbersome, I believe that it was effective in preparing me for upper level classes and helping me to better understand the process of scientific research.”
“I personally felt as though the lab helped me prepare for my other lab courses in chemistry. My ability to be more precise and accurate has dramatically increased since CMB lab.”
In conclusion, we have developed a lab curriculum for lower-level undergraduates that breaks the semester in half, allowing students to carry out two project-based lab sequences. This curriculum is geared toward development of a variety of skills required for successful scientific research, including technical skills (exposure to various techniques), organizational skills (preparation of flow charts), and analytical and communication skills (lab reports). The data presented here suggest that this curriculum has been successful in improving these skills in students.
ACKNOWLEDGMENTS
We thank David L. Smith, Sherilyn G.F. Smith, and David R. Mitchell for support and input.



