Video Views and Reviews: Gastrulation and the Fashioning of Animal Embryos
Even the most naive student readily understands that following fertilization, a single-celled egg must undergo multiple rounds of cell division to become a multicellular organism. In contrast, it is not obvious how the embryonic mass of cells resulting from cleavage becomes transformed into a multilayered, functionally complex, and, in many instances, bilaterally symmetrical embryo. This transformation is so universal among animal embryos that developmental biologists refer to the process with a single term:“ gastrulation.”
Dramatic changes in embryonic complexity accompany gastrulation. The process is all the more impressive for the relative rapidity of its passage and for its pivotal role in bringing about subsequent morphogenetic events in the life history of an animal. Indeed, as one developmental biologist has remarked, “It is not birth, marriage, or death, but gastrulation which is truly the most important time in your life” (Wolpert, 1991).
During gastrulation, many if not all of the newly cleaved cells dramatically change their location within the embryo in a variety of different ways, sometimes individually and sometimes as contiguous sheets of cells. New biochemical influences and physical interactions accompany these changes in cellular neighborhoods. Students studying the underlying cellular details of gastrulation might be encouraged to first examine the process in the development of one organism and analyze the various features of cellular movement. Then they could formulate predictions of how those features might change during gastrulation in other organisms.
With the widespread use of fluorescence microscopy and such fluorescent probes as green fluorescent protein (GFP), a renaissance in the study of cellular movements during gastrulation in living embryos began in the latter part of the twentieth century. These studies have been extensively documented in a recent monograph that describes the molecular interactions that cause and, in turn, result from gastrulation in embryos from many different animal taxa (Stern, 2004). I review here several videos published as freely accessible supplements to this monograph at www.gastrulation.org along with another recently published video of gastrulation in C. elegans. I welcome your comments and questions regarding this review.
GASTRULATION IN SEA URCHINS
Historically, undergraduates taking a course in developmental biology have been introduced to gastrulation using sea urchin embryos, which are small, transparent, and easily preserved for whole-mount observation at intermediate levels of magnification. Thus, the cellular details of the process are readily apparent, as illustrated in the simple drawings of Figure 1 and reviewed by McClay et al. (2004). Live sea urchin embryos are also readily studied using phase-contrast or differential interference microscopy (DIC), and the three movies accompanying the McClay et al. review are exceptionally clear and well resolved; unfortunately, no spatial scales or, for two of the videos, temporal scales are provided by the authors.
Sea urchin gastrulation consists of two or more continuous but somewhat distinct events. During the first phase, small cells called micromeres ingress individually from their location in the outer layer of the hollow, fluid-filled embryo (Figure 2A) into the interior of that embryo (Figure 2B, C). The progeny of these micromeres will secrete the larval skeleton. The individual behavior of ingressing cells is easier to observe in micromeres that have been labeled with GFP, and these are exhibited in a dual video sequence showing, respectively, fluorescence and a combination of fluorescence and DIC (Movie 9_2). Students should examine this sequence repetitively and be encouraged to identify changes in cellular behavior that might be responsible for the process. Are changes in cell adhesion involved? If so, what sorts of integral membrane proteins might be changing their properties? What “forces” the micromeres to ingress? Alternatively, what might prevent their egression? Do these cells also change their shape and motility? These and other questions are easily raised from viewing this initial phase of sea urchin gastrulation. More refined or subtle variations can be explored in subsequent events and during gastrulation in other animals.
During the second phase of sea urchin gastrulation, the epithelial region previously occupied by ingressing micromeres folds inwards and becomes elongated in a process called “invagination” (Figure 2D-F). The inward epithelial fold more accurately resembles the tube one's finger would form by being poked into a balloon. Inquisitive students will ask how invagination begins, and they should be encouraged to consider whether the changes in cellular behavior responsible for ingression could also be responsible for invagination. Might other factors, such as osmosis, be involved? How, for example, is invagination, rather than evagination, produced? As gastrulation proceeds, this finger of epithelial cells continues to invaginate, and the cells at the tip of the invagination begin to sprout long projections called“ filopods” (see Figure 1D). (With a certain degree of imagination, such projections can also be seen in Figure 2F, but unfortunately the video clip doesn't show the completion of gastrulation.) The filopods make contact with the overlying epithelial layer, and gastrulation is said to be complete when the tip of the tube touches the overlying epithelium and fuses with it, producing the larval alimentary system (or“ archenteron”). How might the filopods contribute to the final stages of sea urchin gastrulation? Answers to this and other questions, and underlying molecular and signaling mechanisms, are thoroughly discussed in the McClay et al. review (2004).
Figure 1. Lateral view of early sea urchin development. An embryo containing 16 cells is seen in its entirety in A, and later stages are seen in vertical sections in B-E. In A, four cleavages have produced three tiers of unequally sized cells, with four relatively small cells (or micromeres) at the bottom of the embryo. Micromere cytoplasm remains segregated, and several hours later subsequent cleavages have produced a hollow, fluid-filled embryo (B) with progeny of the micromeres located as indicated. Early and late phases of gastrulation are depicted in C and D, and a highly schematized larva with a complete alimentary system is illustrated in E.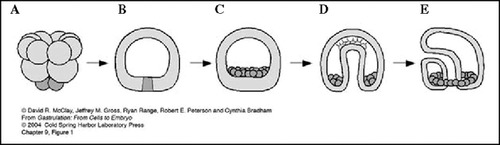
In sea urchins and other transparent embryos containing little yolk, the major consequence of gastrulation for future developmental events is most obvious. Before gastrulation begins, the hollow blastula contains an outer epithelial layer of cells; at the end of gastrulation, the embryo consists of cells segregated into three so-called “germ layers”: an outer“ ectoderm,” an inner “endoderm,” and an intermediate layer called the “mesoderm.” Following the appearance of the three layers, cells will differentiate into major tissues and organs of the maturing larva. Specifically, with regard to derivatives of the endoderm, the first opening that appears with invagination will become the larval anus, the epithelial tube will subsequently be divided into chambers and become the larval gut, and the second opening that forms after fusion of the invaginating tube will become the larval mouth. This developmental sequence appears in other Echinoderms and in Chordates, and for this reason these organisms are said to be “deuterostomes” (literally, “second mouths”).
GASTRULATION IN AMPHIBIANS
With large, yolky eggs, amphibian embryos develop more slowly than sea urchin embryos (raised at similar temperatures), and just as the amount and distribution of yolk affects the size of embryonic cells and their rates of division (Balfour's rule; see Waddington, 1957), this fatty material also apparently affects the mode of amphibian gastrulation (Keller and Shook, 2004). The presence of yolk also makes the fertilized egg and cleaving embryo opaque to microscopic examination, and only surface cell movements during gastrulation can be directly observed on living embryos; interior displacements must be inferred from fixed and sectioned material or examined in live explants. Amphibian gastrulation is not an easy process to investigate or understand.
In spite, or possibly because, of this difficulty, Keller and Shook (2004) discuss gastrulation in amphibians thoroughly and in some detail, with numerous multicolored figures. Their article is accompanied by videos of cell movements in whole embryos and explanted regions of Xenopus laevis (an Anuran) and Ambystoma mexicanum and A. maculatum (Urodeles), and a separate chapter is devoted specifically to discussing different types of cell movement (Keller and Davidson, 2004). Authors' Movie 13_1 depicts surface movements and changes in embryonic shape of a Xenopus embryo over a 15-hour period from the beginning of gastrulation through the formation of the neural tube. Gastrulation in A. mexicanum and A. maculatum are presented in authors' Movie 13_ 9 and 13_10, respectively, and the other 11 video sequences record cell behavior in gastrula explants from both species. Figure 3 presents three stills excerpted from Movie 13_1.
Students watching the videos of amphibian gastrulation will readily appreciate that the process entails cell movement from the exterior surface of the embryo into the interior, but the amphibian process clearly differs from what occurs in sea urchins. While cells enter the interior at a single location, the blastopore, in both groups of organisms, the amphibian embryo is not hollow, and the inward movement of amphibian surface cells does not occur by invagination. Rather, smaller, pigmented cells located at the upper (dorsal) surface of the amphibian blastula begin to spread. The spreading, called “epiboly,” is directed toward a specific region located just ventral to the equator of the embryo, where the cells begin to enter the interior by a process called “involution.” Involution of small pigmented cells creates a dark crescent amid the larger and yellow (yolky) cells (Figure 3A). As epibolic pigment cells converge on this region from more lateral regions of the blastula, the crescent begins to expand (Figure 3B, C), with cells spreading around the entire circumference of the embryo converging to create a continuous circular rim of involution surrounding a protruding group of larger, yolk-filled cells (Figure 3D).
Figure 2. Gastrulation in a sea urchin embryo. The fluid-filled, multicellular stage, or blastula, is depicted during the first phase of gastrulation in A-C, when epithelial cells from the outer layer detach from their neighbors and begin moving into the central cavity in a process called “ingression.” Early features of the second phase of gastrulation — invagination— are depicted in C-E. The transparent embryo is presented in an optical section created by DIC microscopy. No spatial or temporal scales are provided for the video sequences in A-C and D-E, which were taken, respectively, from http://www.gastrulation.org/Movie9_1.mov and http://www.gastrulation.org/Movie9_3.mov.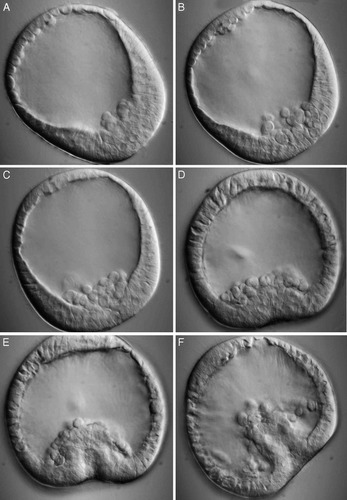
Figure 3. Gastrulation of a yolky Xenopus laevis blastula as viewed obliquely from below. At the start of gastrulation, smaller pigmented cells from the upper surface spread by epiboly, converging on a predetermined region of the embryo and entering its interior by involution. The point of entry is evident as a pigmented rim (A), which gradually enlarges laterally (B and C) as spreading surface cells traveling a longer distance reach the region of involution. Gastrulation is essentially complete when a small plug of large yolky cells is surrounded by a complete rim of much smaller, involuting pigmented cells (D). Gastrulation takes about 7 hours. The time-lapse sequence lasts about 19 seconds and records events lasting about 15 hours; the video is thus presented at about 47 times real time. The video may be viewed online at http://www.gastrulation.org/Movie13_1.mov. Similar sequences involving gastrulation in urodele amphibians (salamanders) are presented at http://www.gastrulation.org/Movie13_9.mov and http://www.gastrulation.org/Movie13_10.mov.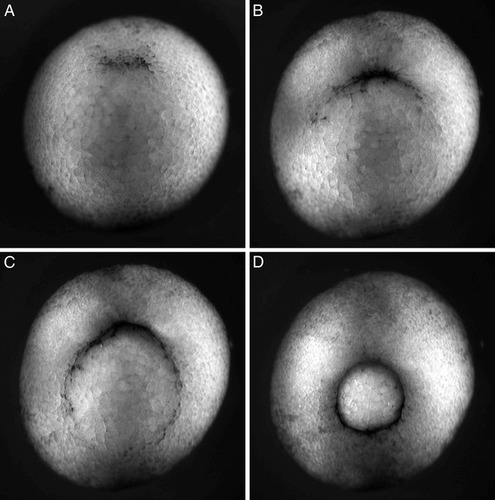
Having examined sea urchin gastrulation and what's known of the underlying cellular mechanisms, students should be encouraged to formulate hypotheses for amphibian gastrulation and how the movements might result from changes in cell shape, adhesion, and motility. Then they can examine the amphibian videos in light of these hypotheses and discuss how involution and epiboly might occur. What causes epiboly? Do spreading cells change their shape? Is a change in shape sufficient, as well as necessary, to explain spreading, or do cells from the interior intercalate with surface cells to increase their aggregate surface area? Are there subtle variations in gastrulation among Amphibia? What additional changes in cell phenotype are required to explain involutional“ burrowing?” What factors regulate involution, and are similar signaling mechanisms responsible for invagination and involution? The video records and accompanying discussion of the various explant experiments, as well as Keller and Shooks' Figures 9, 10, and 12 and accompanying discussion, will test some of their hypotheses and answer some of these questions.
GASTRULATION IN NEMATODES
During the past quarter century, the nematode worm Caenorhabditis elegans (or C. elegans) has become a model organism for studying many questions in developmental biology. Its development is rapid, the eggs are relatively small and transparent (only moderately yolky), and the adults contain a small and constant number of cells, the lineages of which are well known. Our current knowledge of gastrulation in C. elegans is well described by Nance and Priess (2004), with numerous DIC and fluorescent images and colored diagrams.
Figure 4. Laterial view of gastrulation in C. elegans viewed in an optical section created by DIC. Gut precursor cells (“E” daughter cells, labeled with asterisks) are seen initially on the ventral surface of the embryo. As they ingress during gastrulation (B and C), their place is taken by other surface cells (arrows) converging toward the point of ingression. The time-lapse sequence lasts about 7 seconds and records events over a period of 28 minutes; the video is presented at 240 times real time. The video may be viewed at http://www.gastrulation.org/Movie4_1.mov.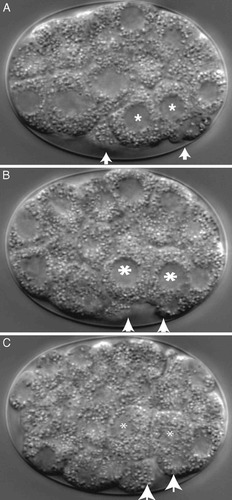
Nematode gastrulation is said to involve ingression and epiboly, and students who are already familiar with these processes from their study of sea urchin and amphibian gastrulation could hypothesize how these processes might occur in an embryo containing only 26 cells (rather than numbers about two orders of magnitude greater in sea urchin and amphibia gastrulae). Will cell number affect these events? If so, how? Following this speculative discussion, the video accompanying the chapter by Nance and Priess (2004) could be examined at http://www.gastrulation.org/Movie4_1.mov, which unfortunately illustrates ingression but not epiboly.
The ingression of two “E” cells in a 26-cell embryo is depicted in Figure 4, which is excerpted from authors' Movie 4_1. The progeny of these cells will give rise to the nematode's gut, so in this instance ingression is analogous to sea urchin invagination and amphibian involution. (Separate ingression of mesodermal and germ-line precursor cells occurs later, but these events are not depicted in the video clip.) As E cells ingress, they are covered by adjacent cells spreading over their outer surfaces, as indicated in Figure 4.1B. Watching this video clip several times, observant students might wonder whether two cells burrowing by ingression into such a small embryo surrounded by an outer egg shell might “force” neighboring cells over their exterior surfaces. Alternatively, they might argue that peripheral cells creeping over the outer surface of the embryo might “force” E cells into the interior. These alternatives can be explored and resolved by reference to isolation experiments described by Nance and Preiss (2004), especially their Figure 4, and by Lee and Goldstein (2003) in a video clip at http://dev.biologists.org/content/vol130/issue2/images/data/307/DC1/Figure.mov.
What cellular mechanisms might be responsible for ingression of E cells in C. elegans? In addition to changes in cell surface properties discussed above, the video clips clearly indicate that burrowing cells change their shapes. Do they move, however, in the same way that cells such as macrophages and fibroblasts move (see, e.g., Lodish et al., 2004)? Lee and Goldstein (2003) also address this question in a series of experiments designed to test the applicability of the fibroblast model. Their article is accessible to most intermediate and advanced undergraduates, who should be encouraged to view the authors' videos and discuss possible molecular models — e.g., actin assembly, actomyosin interactions — and how to test them. Then, students could examine the experiments involving inhibitors the authors performed to explore how ingressing cells move during gastrulation.
ADDENDA
I received two e-mails concerning my recent review of cytokinesis videos (Watters, 2005). One expressed disappointment that the review “overlooked” an article and accompanying videos concerning the use of an extensive RNAi screen to examine cytokinesis in Drosophila (Echard et al., 2004). Unfortunately, the article in question was published a month after I submitted my review for publication, and the accompanying video records were unavailable for study by nonsubscribers for a year from publication date. Although my reviews are not meant to be comprehensive, the results of this screen and the accompanying video records would have rounded out my discussion of other video records describing the role of “sticky.” The videos accompanying the Echard et al. paper became accessible to nonsubscribers after September 2005 ( http://www.current-biology.com/cgi/content/full/14/18/1685/DC1/).
In response to my concern that mitosis not be redefined to include cytokinesis, I was reminded that E.B. Wilson had included cytokinesis (and karyokinesis) as parts of mitosis in the early part of the twentieth century. My goal in expressing this reservation was to acknowledge that many modern introductory biology texts (vide Alberts et al., 2004) now use “mitosis” and“ cytokinesis” to distinguish different events in cell division— chromosome separation and division of the cytoplasm, respectively. I was (and remain) concerned that students might consequently find the inclusion of cytokinesis within mitosis confusing.