A Microcosm of the Biomedical Research Experience for Upper-level Undergraduates
Abstract
The skill set required of biomedical researchers continues to grow and evolve as biology matures as a natural science. Science necessitates creative yet critical thinking, persuasive communication skills, purposeful use of time, and adeptness at the laboratory bench. Teaching these skills can be effectively accomplished in an inquiry-based, active-learning environment at a primarily undergraduate institution. Cell Biology Techniques, an upper-level cell biology laboratory course at St. John Fisher College, features two independent projects that take advantage of the biology of the nematode Caenorhabditis elegans, a premier yet simple model organism. First, students perform a miniature epigenetic screen for novel phenotypes using RNA interference. The results of this screen combined with literature research direct students toward a singe gene that they attempt to subclone in the second project. The biology of the chosen gene/protein also becomes an individualized focal point with respect to the content of the laboratory. Progress toward course goals is evaluated using written, oral, and group-produced assignments, including a concept map. Pre- and postassessment indicates a significant increase in the understanding of broad concepts in cell biological research.
INTRODUCTION
The process of scientific research is a cyclical endeavor that contributes to a general understanding of the natural world. Science produces an increasingly large base of knowledge; however, this base will always contain gaps. Gaps in human understanding solicit questions, hypotheses, and experiments. Experiments generate results and interpretations of results in the context of pre-existing knowledge. The completion of the cycle further adds to human understanding (Achinstein, 2004).
The experimental processes and tools used by scientists proliferate as technology improves, and this results in an increase in the resolution of human understanding. From an academic bias, this causes compression in which it becomes more difficult to prioritize which scientific skills and content need to be taught within a given period, such as a college semester (Harwood, 2003). The relationship between concepts and skills becomes strained as both need to be introduced earlier within an academic or scientific career. What has emerged from this conflict is that teaching students the scientific process as a means of covering content has become increasingly popular, and various authors have recently addressed the changing landscape of training in the biomedical sciences (National Research Council, 2002), and specifically in cell biology (DiCarlo, 2006).
Two cycles work within basic and clinical biomedical research. As described, the scientific method is a cyclical process that produces knowledge. A second cycle combines modern fiscal realities with the dissemination of experimental findings and new knowledge. In this cycle, preliminary results in the context of background knowledge provide the framework for a request for resources for further research. Resources allow experiments, which produce results and interpretations. New knowledge is disseminated to the provider of the resources (often the public), thereby justifying more resources.
A growing trend in biomedical pedagogy is to implement inquiry-based courses and laboratories for undergraduate students (Hake, 1998; Bonner, 2004; Handelsman et al., 2004; Oliver-Hoya, 2004; Howard and Miskowski, 2005; Lynd-Balta, 2006). Can Caenorhabditis elegans (C. elegans; a free-living nematode further described below) be exploited to teach undergraduates multiple aspects of biomedical research? I've developed an upper-level laboratory course that takes advantage of nematode biology and introduces students to both the theoretical/experimental and fiscal/dissemination cycles within biomedical research. Learning activities include two half-semester independent projects intermingled with technique-focused individual laboratories. Major assessments include a written proposal, a concept map, and an oral presentation. Within this article, I describe and analyze this laboratory course and its assessments.
MATERIALS AND METHODS
C. elegans Culture, Strains, and RNA Interference (RNAi)
Nematodes were cultured using standard techniques (Brenner, 1974; Stiernagle, 2006). The rrf-3 (pk1426); him-5 (e1490) strain was constructed in the laboratory of S. Emmons (Albert Einstein College of Medicine) and obtained through D. Portman (University of Rochester Medical School). A library of bacterial feeding RNAi clones was obtained from the MRC Geneservice (www.geneservice.co.uk/home). Bacterial feeding RNAi was performed using modifications of existing protocols. First, individual Escherichia coli HT115 (DE3) strains harboring RNAi clones were picked from library plates and grown on solid Luria Bertoni (LB) supplemented with 200 μg/ml ampicillin at ∼20°C. Colonies were grown overnight in liquid LB supplemented with ampicillin and 20 mM glucose. A small volume (10–100 μl) of saturated overnight culture was plated on M9-lactose plates (20 mM Na2HPO4, 20 mM KH2PO4, 10 mM NaCl, 20 mM NH4Cl, 0.5% casamino acids, 2.5% agar). Embryos were harvested from gravid hermaphrodites by gentle hypochlorite treatment (Stiernagle, 2006) and ∼200 plated along with RNAi bacteria. Alternatively, 5–10 gravid adult hermaphrodites were plated after multiple washes with sterile water. The SU93 strain of C. elegans contains a fusion between green fluorescent protein and AJM-1, a resident protein of apical junctions between epithelial cells (Mohler et al., 1998; Köppen et al., 2001) was obtained from the C. elegans Stock Center (www.cbs.umn.edu/CGC). More detailed protocols and positive control bacterial feeding RNAi strains are available upon request.
Molecular Biology and Concept Map Assignment
RNAi library clones are contained in the vector pPD129.36 (Kamath et al., 2000). The sequences of the primers used to amplify the genomic insert were pPD129.36 forward 5′-GTGAGCGAGGAAGCAACCTGG-3′ and pPD129.36 reverse 5′-GTAAAACGGCCAGT-3′. DNA purification kits were obtained from Omega-Biotek (Doraville, GA), and they were used according to manufacturer's instructions. Restriction enzymes, ligase, and competent bacterial cells were obtained from New England Biolabs (Ipswich, MA), and they were used according to manufacturer's instructions. Other molecular biological reagents/protocols were standard. Students were given time credit toward the minimum of 10 h for each step of the molecular biology project according to the following: plasmid miniprep, purifying a polymerase chain reaction (PCR) product or gel extraction = 0.5 h; agarose gel electrophoresis = 1.5 h; setting up a PCR reaction = 0.5 h; setting up a restriction digestion or ligation reaction = 0.5 h; and bacterial transformation = 0.5 h. These eight enzymatic manipulations or purification protocols are the concepts that students link together into a concept map.
Immunocytochemistry
The freeze-crack, cold methanol method of fixation was used to prepare worms for antibody staining (Hurd and Kemphues, 2003). The monoclonal antibody (mAb) MH27 recognizes AJM-1 (Francis and Waterston, 1991). The mAb E7 recognizes β-tubulin (Chu and Klymkowsky, 1987) and reliably stains embryonic mitotic spindles with the standard freeze-cracking method. The mAb AA4.3 recognizes α-tubulin (Walsh, 1984) and stains embryonic mitotic spindles with a modified freeze-cracking method in which Ruvkun's fixative (Finney and Ruvkun, 1990) is substituted for methanol during fixation. All three were obtained from the Developmental Studies Hybridoma Bank (http://dshb.biology.uiowa.edu/). Secondary antibodies were purchased from Jackson ImmunoResearch Laboratories (West Grove, PA).
Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE)
Protein electrophoresis was done according to standard procedures (Laemmli, 1970). GelCode Blue (Pierce Chemical, Rockford, IL) was used to stain for total protein in the gel.
Analysis of Oral Presentations (Question, Approach, Experiment, Result, Literal Interpretation, and Speculative Interpretation [QAERLS]) and the Entry and Exit Questionnaire
Identical 10-question entry and exit questionnaires were administered during the first and last session of the laboratory. Students were instructed to not supply a name, and they were told that it did not play a role in the grade for the course. For each year, entry and exit questionnaires were mixed together and graded blindly. Means and standard deviations were calculated and compared using an unpaired, two-tailed Student's t test (www.physics.csbsju.edu/stats/ttest.html). Each student's ability to identify and explain a biomedical question, approach, experiment, result, literal interpretation, and speculative interpretation were assessed by the instructor during the oral presentation sessions in all years and also by other students in the 2005 cohort.
COURSE GOALS AND OUTCOMES
Cell Biology Techniques (BIOL-311L) is the laboratory corequisite to Cell Biology (BIOL-311). This combination is the last in a series of core biology courses at St. John Fisher College. Students receive two credits and meet for 4 h once a week (the actual time met per week can be variable; see below). Four broad learning goals of the laboratory were established with the idea of training future biomedical researchers.
On completion of Cell Biology Techniques, students will be able to
Appreciate the multidisciplinary nature of cell biology
Identify and utilize the scientific method
Work competently and comfortably in a cell/molecular biology laboratory
Evaluate and communicate scientific experiments, results, and knowledge.
During the course of the semester, students work toward achieving these goals through a number of specific assignments and lab activities, each of which produces a measurable outcome. For example, students synthesize previous research, and produce and deliver a PowerPoint presentation and prepare a written proposal for future research upon a gene/protein, both of which support goals 1 and 4 (the assignments and evaluation criteria are part of Supplemental Material). Students handle microorganisms (nematodes and bacteria), perform epigenetic experiments, and document phenotypes by using multiple forms of light microscopy. These outcomes allow for the assessment of goals 2 and 3. Finally, students carry out a series of molecular biology techniques while documenting and evaluating their progress in a laboratory notebook. This provides an opportunity to evaluate progress toward achieving goals 2–4. In addition to these activities and outcomes, another objective of this laboratory class is to allow students to experience a novel discovery within the iterative nature of the biomedical research process. This is addressed by the overall design of the lab, in which students pursue independent and unique projects that require an empirical assessment of success and often need repetition.
Course Rationale
Modern molecular and cellular biology encompasses the observation and manipulation of organisms, cells, and the biological macromolecules DNA, RNA, and protein both in vitro and in vivo. When considering the design of Cell Biology Techniques, I sought to allow students to discover the hierarchical relationships among these biological entities (Khodor et al., 2004), rather than simply teach them a series of techniques. However, the complexity of individual eukaryotic cells and metazoan organisms makes complete understanding the central challenge of cell biology research and instruction. This challenge is amplified in an undergraduate institution because most students seek biological understanding of the organism with which they are most familiar, Homo sapiens. Given these considerations, I chose to center the course on an extremely simple organism, the nematode roundworm C. elegans, to allow undergraduates to manipulate and explore the relationships among biological entities.
A second rationale for the design of Cell Biology Techniques was to allow students unstructured time during the semester in an attempt to teach and observe their time-management skills and to allow for repetition of protocols. To be successful, active biomedical researchers (or practitioners of any other vocation) must learn to manage time effectively and balance multiple projects and responsibilities. In the course of the two independent projects during the semester, students are given free time to pursue their projects at their own pace. To assess progress, checkpoints are used. To facilitate success, multiple attempts at any particular protocol are encouraged.
Laboratory Components and Results
RNAi Screening in C. elegans.
C. elegans is a free-living nematode that was chosen as a model organism to study developmental and neurobiological processes. A host of attractive features make it an ideal organism in an undergraduate setting, including inexpensive and simple culture; a transparent, simple anatomy; well-established genetics; and efficacious, powerful epigenetic techniques such as RNAi. RNAi is a conserved mechanism of epigenetic inhibition of gene expression first characterized in worms (Fire et al., 1998), and subsequently observed in a diverse array of organisms. It has been used by many groups as a method to link abnormal phenotypes to genes across a part or a whole genome, and it has also been suggested to be of therapeutic value (Dykxhoorn and Lieberman, 2005; de Fougerolles et al., 2007). C. elegans offers an array of methods to deliver double-stranded RNA and to observe phenotypes caused by inhibition of gene expression (Kim, 2001).
Bacterial feeding-based RNAi is an established epigenetic technique used to reduce the expression of genes in C. elegans (Kamath et al., 2000; Simmer et al., 2002; Sonnichsen et al., 2005). Effective and specific RNAi of single genes involves nothing more than aseptic technique, and it has been used in large-scale projects by numerous labs (Kamath et al., 2003). The simplicity and cost-effectiveness of this epigenetic technique suggested that it was amenable to an inquiry-based lab at a primarily undergraduate institution (Sundberg et al., 2000) and that it would allow undergraduates to make novel observations and formulate their own questions and hypotheses. Inquiry-based laboratory activities have been shown to increase retention of content and overall attitude toward science at multiple educational levels (Hake, 1998; Bonner, 2004; Handelsman et al., 2004; Oliver-Hoya, 2004; Howard and Miskowski, 2005; Lynd-Balta, 2006).
During the first half of the 12-session laboratory students learn basic C. elegans culturing techniques, microscopy, and nematode anatomy. These techniques, along with previously learned microbiological sterile technique, allow students to perform a bacterial feeding RNAi screen for novel phenotypes. Each student selects five genes from a library of RNAi clones (Kamath et al., 2003), and then he or she performs RNAi by using a hypersensitive strain of C. elegans (see Materials and Methods). The selection of clones is made using available published RNAi data found in the supplement to Simmer et al., (2002) and is available on WormBase (www.wormbase.org). Students post their selections online to a discussion board, which nearly eliminates overlap. Two differences separate this screen from prior large-scale RNAi screens. First, the use of embryos harvested after bleach treatment makes this primarily a postembryonic screen. Second, in addition to RNAi hypersensitivity, the worm strain also carries a mutation that causes a high incidence of males (Him). Larval/adult and male phenotypes are largely unexplored using RNAi. These differences are highlighted and used to motivate students to make original scientific observations. Before selecting genes from the library, students perform positive control RNAi experiments. In the hands of the students, RNAi of par-1 causes a severe and reproducible protruding vulva phenotype (Hurd and Kemphues, 2003), and RNAi of tbb-2 causes slow larval growth and eventually larval arrest (unpublished data). Although not quantified, the majority of these control experiments show the expected results year after year.
Because repetition of a particular protocol enhances the chances for success, students screen their five genes in an initial set of two experiments with controls and then a subsequent set of three with controls. If an RNAi experiment in their initial set failed due to contamination, or if an obvious phenotype was observed, the second set can be expanded to their full five clones with controls. Repetition of experiments provides rigor, but it is also costly (Sundberg et al., 2000). Although prior screens have used isopropyl β-d-thiogalactoside, lactose provides an inexpensive and effective inducer of transcription of the RNA in the bacterial cells (see Materials and Methods). Performing positive controls that produce easily scored phenotypes and a negative control that yields wild-type adults also increase the repetitions. In the Fall 2006 semester, 35 students started 214 overnight bacterial cultures that led to 137 successful experiments. A successful RNAi experiment included a minimum of 100 total worms and lacked contamination on day 3. Contamination (usually by mold) is the most common reason that an individual experiment is declared a failure.
During the past 3 yr, students have observed both expected and novel phenotypes. For example, RNAi depletion of ribosomal proteins causes slow growth, larval lethality, or both, which provide direct experimental reinforcement of lecture content (translation is an essential process). As another example, unc-52 encodes a component of the basement membrane between muscles and the hypodermis. Postembryonic RNAi causes fully penetrant and highly expressive paralysis, which reinforces the idea that the extracellular matrix is essential in a metazoan. Although ZC123.3 (RNAi) has been shown to cause Pvl in the embryonic screens, a similar postembryonic RNAi result narrows the focus of its role (Figure 1). ZC123.3 encodes a homeobox, zinc-finger–containing protein. Finally, C45G3.1(RNAi) causes a loss of male tail rays. It encodes a protein thought to control cell proliferation in neuronal lineages in mammals (Bond et al., 2002).
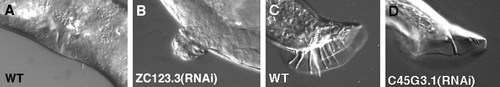
Figure 1. Expected and novel phenotypes revealed by postembryonic RNAi of C. elegans in the context of an undergraduate laboratory. (A) Lateral view of a wild-type adult C. elegans vulva. (B) Abnormal vulva discovered by a student that was caused by depletion of ZC123.3. (C) Lateral view of a wild-type adult male tail (nine sensory rays per side). (D) Abnormal male tail discovered by a student (fewer rays and misshapen fan) that was caused by depletion of C45G3.1. Both of these phenotypes were reproducible, highly penetrant, and expressive.
The five-gene screen that each student performs often yields an abnormal phenotype. Students that do not observe an RNAi phenotype still choose a single gene from their pool of five to become their “gene of interest.” This gene/protein becomes the subject of their proposal and oral presentation. With some coaching, this decision is usually based on the criteria that something significant has been discovered about a homologue. An interpretable experimental result is the subject of their presentation (further described below), and this experimental result could be their own RNAi result or a published result. Because initial pools of five genes were nonoverlapping, each student ends up with a different gene of interest.
The RNAi screen also allows students to enter into the two cycles that drive modern scientific advance. First, a theoretical-experimental cycle exists in which background (B) knowledge, which contains gaps, suggests a question (Q). In most worm genes, other RNAi screens or analysis of mutants has often yielded some information about cellular role. A general approach (A) is devised, a hypothesis may be proposed, and a specific experiment (E) is performed to address the question/hypothesis. In this case, it is the student's postembryonic RNAi experiment. Results (R) of the experiment are collected and interpreted on two levels. First, a literal interpretation (L) provides a simple and logical extension of the results; it is often a conclusion. Did postembryonic RNAi cause an abnormal phenotype, such as lethality or infertility? If so, the gene is required. In addition, authors often put forth a more speculative (S) interpretation that attempts to fit the results into the context of background knowledge. What is the molecular identity of the gene? Does the abnormal phenotype tell us something about the cellular role of the protein? The abbreviation BQAERLS summarizes this cycle, and it was originally taught to me by Dr. William M. Saxton and Dr. J. Jose Bonner at Indiana University during a first-year graduate seminar about critical reading of scientific literature.
The theoretical-experimental cycle is superimposed on a cyclical, modern fiscal reality lived by biomedical researchers as described above. The points of evaluation inherent to the fiscal cycle provide a means for assessment in the context of an undergraduate teaching laboratory. Students produce a written proposal for further experimentation and an oral presentation about the gene they have chosen to be of interest. These are assessed using grading rubrics (Allen and Tanner, 2006) made available (Supplemental Material).
The written proposal is designed to provide an opportunity for students to think about the next logical experiment in a series of experiments to understand the role of a particular protein. Preliminary data for their proposal is gathered from the plethora of online databases and tools available for C. elegans (Antoshechkin and Sternberg, 2007). It is written in a style reminiscent of National Institutes of Health grants. The first specific aim is actually the RNAi experiment that the students have already performed (I ask them to pretend to go back in time to the beginning of the semester). The second aim is often hinged upon the biochemistry or genetics of the gene/protein that they have chosen. Choosing this second aim reinforces lecture content because it follows the introduction of many modern molecular approaches to cell biology. For example, students might propose a two-hybrid approach if the identity of their protein suggests physical interactions with other proteins. They might propose to make a green fluorescent protein (GFP) fusion if the anatomical or subcellular localization of their protein is unknown. The proposal is assessed using grading criteria made available (Supplemental Material).
The oral presentation accounts for approximately one-third of the grade for the semester. The requirement for the oral presentation is a completed BQAERLS (Supplemental Material) for a single experimental finding. It might be their RNAi result, or it might be a published result that concerns a homologous gene/protein. The purpose of the oral presentation is to allow students to discover content through skills. Each student becomes an expert in the cell biology of the particular gene/protein that they have chosen, and all get a chance to practice oral presentation skills. They present in sessions of 8–10 students, which creates an informal and supportive situation in which they can learn from each other. Audience participation in these sessions is guaranteed as each student is required to ask at least two questions during the session. Asking more questions, if relevant and significant, can earn a student some extra credit.
Which aspects of the described scientific method do students find the most difficult to fully comprehend and present? Because available background (B) information for each different gene may be quite different, it was not included in the analysis. The other six steps of the scientific method as described above (QAERLS) were each assigned the same total point value (5), and averages from three different cohorts are presented in Figure 2. These data indicate that in general, students understand this form of the scientific method and can present data to peers and their instructor. Although not statistically different, the lowest scores are often achieved during the presentation of experimental results (R). This was surprising as I hypothesized at the beginning of using this assignment 4 yr ago that students might struggle the most with the speculative nature of the author interpretation (S). Using the same rubric, students tended to give each other slightly higher scores than given by the instructor (Figure 2D).
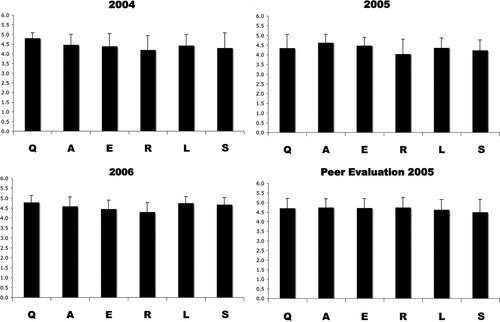
Figure 2. Student oral presentation performance. Average scores for each cohort for the years 2004–2006 are shown for student oral presentations. For 2005, both instructor evaluation and peer evaluation are shown. Student's abilities to explain the question (Q), approach (A), experiment (E), result (R), literal interpretation (L), and speculative interpretation (S) of a piece of cell biological data were quantified using the Presentation Evaluation rubric (see text and Supplemental Material).
Molecular Subcloning.
No matter how students chose a gene of interest, during the second half of the semester, they attempt to subclone the genomic DNA insert contained in the RNAi library vector by using the tools of molecular biology. First, students purify the library clone plasmid (miniprep) from bacterial cells grown during the RNAi screen. Then, they amplify the insert by using PCR and primers that recognize sites in the vector. Successful PCR reactions are then purified and digested using appropriate restriction enzymes. Successfully digested insert DNA is then gel extracted. When this point is reached and documented, students receive a bacterial pellet that contains the destination plasmid vector. The destination vector is miniprepped, digested, and gel extracted in a manner very similar to the insert, providing another opportunity for repetition of protocols.
Molecular (DNA) cloning is a staple of modern biological analysis. It is conceptually simple, yet each step is subject to numerous technical pitfalls, and these provide multiple opportunities for students to analyze their own technique, optimize, and troubleshoot. The process of cloning is initially presented to the students as a linear progression from DNA of interest to useful clone, but the primary goal is to have students understand the relationships among the tools and to see the tools as an interconnected network. The indeterminate and technically challenging nature of even simple molecular cloning makes assessing student achievement based directly on their progress nearly impossible. I have tried two approaches to controlling how much effort each student puts into this project, and I have used a concept map as the main summative assessment.
The first of the two approaches used in 2005 was to simply let the students decide, in consultation with the instructor, when continuing the cloning project was essentially hopeless. This point was reached when the chance of future progress involved many steps backward and the semester was nearly over. Initial incentives for the students were indirect in this year. First, they knew that their notes were going to be turned in and evaluated, not for completion of the project, but rather for note-taking technique. Second, I mentioned often during this half of the lab that success in this project would provide positive subject matter for a future letter of recommendation. Under these incentives, students devoted an average of 8.3 h, and most progressed to the stage of digestion of insert DNA. Three students “finished” the project by reaching the stage of transforming a ligation reaction into competent bacterial cells (Table 1). These three students devoted 10.5, 11, and 12 h each.
| Procedure | 2005 | 2006 |
|---|---|---|
| Miniprep of library clone | 30 + 10 | 35 + 20 |
| PCR insert | 30 + 17 | 35 + 24 |
| Purify PCR product | 24 | 24 |
| Digest insert DNA | 24 | 3 |
| Gel extract insert DNA | 10 | 3 |
| Restrict destination vector | 5 | 3 |
| Gel extract vector DNA | 3 | 2 |
| Ligate insert and vector | 3 | 0 |
| Transform ligation | 3 | 0 |
The second approach used in 2006 was to give time credit (see Materials and Methods) for each of the techniques involved in molecular cloning and to assign each student a minimum of 10 h on the project. The rationale behind this was twofold. First, the prior year average of 8.3 h did not seem like enough effort; and second, given the other activities undertaken during the second half of the semester (see below), 10 h were available within the number of weeks of 4-h laboratories. Under this scenario and also using the indirect incentives mentioned above, no student made it to the transformation step, and very few made it past digestion of their insert DNA (Table 1).
To assess understanding of molecular cloning and the appropriate use of the main tools of molecular biology, teams of four students produce a concept map (Allen and Tanner, 2003; Novak, 2003). The concepts are the techniques, and the assignment is to link them into a network with appropriately described, often bidirectional connections. There are 15 such links, so the assignment is worth 15 points, and each missing or inappropriate link reduces the score by 1 point. On the whole, most teams produce very good maps during most years. The average scores over the past 3 yr were 12.3 in 2004, 12.8 in 2005, and 12.3 in 2006. An example of an excellent map is shown in Figure 3(the original PowerPoint file is available in Supplemental Material).
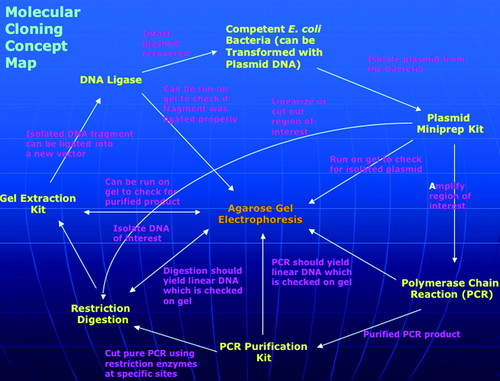
Figure 3. An example molecular cloning concept map produced by students.
Although the usefulness of a successful subclone is likely to be minimal as described below, students maintain a high degree of engagement during the subcloning, and it serves as a entry point to recruit students to pursue independent projects. The destination vectors were designed for the yeast two-hybrid system (James et al., 1996). However, the RNAi library clones are not particularly useful as starting material in this context as they are fragments of genomic DNA. They rarely contain the entire coding sequence and often contain significant amounts of intron sequence (Kamath et al., 2003). Only a handful of students over the past 4 yr have perceived this and raised the question of the utility of the subclone that they might construct. Most students become absorbed in troubleshooting techniques and finishing the project. Although designing this project to yield a useful clone would likely increase student engagement and perhaps apply to research in the same manner as preliminary RNAi results, time and cost constraints are currently inhibitive in the context of a college semester. The design of this subcloning project represents a compromise between pedagogical objectives and pragmatism. Nevertheless, this teaching laboratory exercise in cloning attracts the interest of certain students, and several each year elect to pursue independent research projects that involve molecular cloning.
Technique Laboratories.
Microscopy.
To visualize both normal C. elegans anatomy and abnormal phenotypes, students learn and practice two microscopy techniques in the second lab of the semester. First, the stereodissection microscope is a staple in C. elegans labs, and it allows students to discern males from hermaphrodites, observe normal crawling behavior, and determine fertility of a population. Second, differential interference contrast (DIC) microscopy is also a necessary commodity for worm research because it allows for the observation of anatomical features within the worm's transparent body. Evidence of competence with these two techniques is collected in the form of photomicrographs taken throughout the semester. The second laboratory focuses on normal worms. Pictures of abnormal phenotypes observed during the RNAi screen provide additional assessment of the acquisition of these skills (Figure 1).
The advent of transgenic introduction of fluorescent proteins into various model organisms has revolutionized modern cell and molecular biology (Chalfie et al., 1994). In addition to the two white light microscopy techniques mentioned, students observe strains of worms that have a stable transgenic insertion of GFP under blue light during the third and fourth laboratory sessions. There are several hundred strains of C. elegans available that contain GFP, often fused to a worm protein of interest. From a cell biological point of view, one very useful strain is SU93, which contains a stably integrated fusion of GFP to a protein that is a component of apical junctions (Köppen et al., 2001; Mohler et al., 1998). There are many more such strains available from the C. elegans stock center (see Materials and Methods). The GFP visualization laboratory sessions are also positioned within the semester to reinforce lecture content, and in addition, the beauty of glowing worms produces a “wow factor” among the students.
Immunocytochemistry.
Although GFP has become the method of choice to localize proteins in C. elegans, immunocytochemistry is still used in worms and many other model systems. In a laboratory session during the second half of the semester, students fix and stain worms with antibodies against tubulin or against the same apical junction protein that was visualized with a GFP fusion earlier in the semester (see Materials and Methods). Observation of microtubules with an antibody allows for reinforcement of lecture content as this lab falls during the cytoskeleton unit. Using both an antibody and a GFP fusion to show the accumulation of a junction protein demonstrates to students that there is more than one way to localize proteins in a cell or organism.
SDS-PAGE.
The final laboratory of the semester is a demonstration of protein separation by using SDS-PAGE. Students address the question of whether extracts from different sources have the same collection of proteins. Students are encouraged to create samples of their choice. Over the past few years, student samples have included C. elegans extracts, sheep blood (borrowed from the animal physiology lab), earthworm extract (caught in the wild), various feline organ extracts (borrowed from the zoology lab), and pure protein samples that are available such as immunoglobulins (the primary or secondary antibodies used for immunocytochemistry) or bovine serum albumin. The differences between DNA electrophoresis, which students have done repeatedly by this point in the semester, and protein electrophoresis are stressed as students assemble and pour their own gels. Gels are stained for total protein and visualized within the lab period.
Effectiveness of the Laboratory
To assess the overall effectiveness of Cell Biology Techniques, the following 10-question entry and exit questionnaire is administered in the first and last sessions of the semester. It focuses on major concepts, terms, and the experimental approaches used in multidisciplinary cell biological investigation.
Explain information flow (from DNA sequence to characteristics of an organism) in biology.
Explain the use of model organisms in experimental biology.
Explain the genetic approach to understanding cell biology.
Explain the biochemical approach to understanding cell biology.
Explain the difference between the biochemical function and the cellular role of a biological macromolecule (like a protein).
Explain the difference between a genetic technique and an epigenetic technique.
Explain the difference between the genetic approach and the genomic approach to understanding cell biology.
Name one site on which to find DNA sequence information on the Internet.
Explain the difference between molecular cloning and organism cloning.
Explain the difference between mutant and transgenic organisms.
These questions were each assigned 5 points, and the results for both the 2005 and 2006 cohorts are shown in Figure 4. Statistically significant improvements (p < 0.05) were made by the 2005 cohort for all questions except 1 and 7. Significant improvements were made by the 2006 cohort for all questions except 1, 2, 7, and 9.
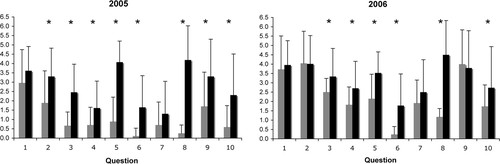
Figure 4. Entry and exit questionnaire performance. Bar graphs depict mean ± SD scores for each question of the entry–exit questionnaire for both the 2005 and 2006 cohorts. Mean entry scores are gray. Mean exit scores are black. Significant differences (Student's t test; see Materials and Methods) are indicated by asterisks.
DISCUSSION
C. elegans provides an ideal, powerful model system for undergraduates to practice cell biology. The laboratory course described in this article takes advantage of nematode biology to teach both cell biological content and scientific skills. The majority of the content of the lab is individualized for each student because it is centered on the particular gene/protein that they chose to study near the midpoint of the semester. This results in a broad variety of molecules of interest to cell biologists being studied each year. Over the past 4 yr students have studied nuclear pore proteins, ribosomal proteins, components of cellular junctions or the cytoskeleton, signaling proteins, extracellular matrix components, and proteins involved in membrane transport. Observation and conversations with students indicate that the chance to choose what to study instills a sense of ownership of the gene/protein they pursue. From the point of view of the instructor, the “random” choice of genes piques interest every year and provides incentive to enable student success. The repeatable preliminary results can be followed in other upper-level labs (e.g., developmental biology) or by a student pursuing an independent project. Modifier RNAi screens should also be possible in an undergraduate setting.
Student performance was measured using both formative and summative assessments. The oral presentation assignment and rubric have provided formative data over 3 yr that have directed me toward helping students fully understand and explain complex experimental cell biological procedures and results. As a summative assessment, it allowed the evaluation of multiple levels in Bloom's taxonomy at the end of the semester (Bloom and Krathwohl, 1956). One caveat to these interpretations is that the BQAERLS divisions are not equal. The amount of available and relevant background information can be drastically different between genes/proteins. In addition, explaining a question and an approach is more straightforward for undergraduates than critically evaluating expert author's interpretations of their own data.
The concept map has been a useful tool to assess student understanding of the molecular cloning project. Although it could be administered both before and after the project (Novak, 2003), I have found it effective as a summative assessment, especially as a group effort among upperclassmen. The average scores over the 3-yr period indicate a fairly detailed understanding of the relations among the techniques of molecular biology, and how molecular cloning is a networked process and not a linear pathway. In addition, it provides a cooperative learning situation, shown to benefit student comprehension at multiple educational levels and in multiple disciplines (Crouch and Mazur, 2001; Patel et al., 2004; Slavin, 1996).
The production of the concept maps is a team effort among four students, a common and effective group size (Antil et al., 1997). In addition, groups are balanced with a mixture of stronger and weaker students. They tend to meet and accomplish this project during nonlab time, but the proximity of the biology computer lab offers the chance to make informal observations while teams work to construct their maps. I have observed that students adopt typical roles in this situation. One person sits at the computer and does most of the construction. The core curricula of both the college and the biology department have required the creation and presentation of PowerPoint slides multiple times before this assignment, so all students have a basic set of skills. Another student typically provides leadership and focus, sometimes by sketching an evolving rough map on the whiteboard. The other two students are involved in brainstorming, debating the linkages between concepts, and checking facts using the Internet or the resources available in the lab (e.g., manuals, other students, the instructor). Although I have repeatedly observed this group dynamic arise in past years, I have also heard that teams have accomplished this project by simply emailing an ever-changing version of their map among themselves until all were happy with the final product.
The open-ended nature of the two major projects allows the opportunity to observe and assess time management skills. I am currently developing criteria with which to evaluate students' use of their time while engaged in the open-ended projects. Preliminary observations of student behavior over the past few years indicate drastically different approaches. Some students pursue both projects in small, even steps whereas others perform them in their entirety in a few last-minute, sometimes late-night binges. The data suggest that mandating a minimum time spent pursuing the cloning project actually decreased the average progression into the project (Table 1). However, a higher percentage of students performed second minipreps and PCR reactions in 2006, which could be due to an increase in errors or bad reagents.
Is this laboratory effective? The data presented in Figure 4 indicate that significant improvements were made in student performance on many questions of the entry/exit questionnaire. Students were able to better describe and differentiate big concepts like the genetic approach (3) and the biochemical approach (4) to cell biological investigation (Wong et al., 2005). In addition, students better comprehend the use of mutations and transgenic technology in model organisms (2 and 10) to understand biology (Fields and Johnston, 2005). However, the data also indicate that the (sometimes subtle) differences between genetics, epigenetics, and genomics are still not well understood, even after being directly exposed to these in the lab (6 and 7). The data also indicate that students enter the lab with a general understanding of the central dogma of biology (1) and cloning (9, especially in 2006). Finally, many suggest that Google is a place to find DNA sequence information on the Internet before taking the lab, and nearly all answer WormBase or the National Center for Biotechnology Information at the end of the semester (8).
Comments on course evaluations suggest that students value the experiences provided in Cell Biology Techniques. For example, they appreciate the ability to work on their own time and at their own pace. One said that “the flexibility of when work could be done” was one aspect of the course that was beneficial to their learning. In addition, many over the past 4 yr have remarked positively about the active-learning, individualized approach by noting the connection between the design of the lab and the “real world.” However, every year a few react negatively to having to troubleshoot their own projects, “some reactions didn't work,” and to the time freedom, “it seemed as if everything happened at the end.” The data presented above in combination with these student comments suggest that the design of this laboratory accurately reflects the nature of scientific inquiry and biomedical research. Alumni that are currently in graduate programs in the natural sciences, in clinical/preprofessional programs, or in secondary education have remarked that they still remember “their gene” from cell biology lab.
ACKNOWLEDGMENTS
I thank Drs. K. F. Picardo and R. M. Miller for critical reading of the manuscript and the Biology Department at St. John Fisher College for insightful discussion, resources, and help. All monoclonal antibodies were obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the National Institute of Child Health and Human Development and maintained by Department of Biological Sciences, The University of Iowa (Iowa City, IA). All C. elegans strains were obtained from the Caenorhabditis Genetics Center developed under the auspices the National Center for Research Resources and maintained at the University of Minnesota (Twin Cities, MN).



