Physics and Biology Collaborate to Color the World
Color may be evolution's most beautiful accident. Cristina Luiggi, writing in The Scientist
To understand how life works, it is essential to understand physics and chemistry. Most biologists have a clear notion of where chemistry fits into their life sciences research and teaching. Although we are physical beings, physics does not always find a place in the biology curriculum. Physics informs and enlightens biology in myriad dimensions (Figure 1), yet many biology courses proceed with little or no consideration of physical properties or principles. Phil Nelson, a biophysics professor at the University of Pennsylvania, asserts, “Physical models are often weirdly, unreasonably effective in stripping away the inessential from a biological system—and in displaying connections between things that seemed not obviously connected to our untrained imagination.” In this review of online media in the realm of biological physics, I will meander up and down the scale of nature to explore the intersection between physics and biology. Let us begin with the macro scale and the most interesting subject of all, ourselves.
Figure 1. Left, a hyperbolic paraboloid. (Courtesy of mathforum.org.) Middle and right, gyroid structures. (Courtesy of Adam G. Weyhaupt, +plus magazine.) As physical structures, these remarkable mathematical constructs are pivotal in understanding aspects of biological color and biomechanics.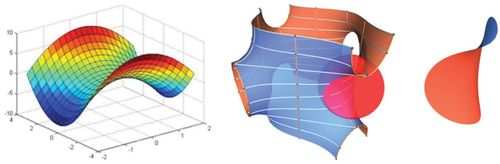
While your cat can likely outrun you in a short- or middle-distance race, you would probably win a longer race. Our species excels at long-distance endurance running, and in fact, running prey to exhaustion is a hunting technique used by wolves and modern humans. Dan Lieberman at Harvard University is a leading researcher in the study of the functional form of the human body. He and his colleagues have a website devoted to barefoot running (http://barefootrunning.fas.harvard.edu), a fad that has pushed the limits on acceptable footwear at the office. How is it that, in our shoeful society, where the average American cannot run a mile wearing shoes, some individuals can run hundreds of miles a month in bare feet? In addition to dodging hazards, a barefoot runner learns to strike the ground first with the forefoot rather than with the heel. Striking with the forefoot dampens the amplitude of high-impact transients that heel strikes generate. The strong impacts generated by heel strikes can also be dampened by the elevated and well-cushioned heels of running shoes, which were first developed in the 1970s (Figure 2). So which is the better way of running? Does one way minimize bodily damage from impact transients better than another? We do not know for sure yet, and students might be interested in learning more about the methods that Lieberman and his colleagues are employing to get answers. You can view good videos illustrating their research by clicking on Biomechanics and Videos at the top navigation bar of their website. However, I recommend first viewing a video produced by Nature to go with a Lieberman journal article (link is available at the bottom of this page: http://barefootrunning.fas.harvard.edu/1WhyConsiderFootStrike.html). It features Lieberman himself barefoot running in Massachusetts, on pavement, in the winter. In 6 min, you will see hypotheses and methods of study, and students should be able to see from the force transduction graphs how different the profile is for a heel strike versus a forefoot strike. There are important biological questions wrapped up in the force mechanics. What are the evolutionary trade-offs in adapting bones to specific functions? How can an organism's power and speed be balanced against mechanical stress and tissue trauma?
Figure 2. Measurements of the forces occurring at the ground for (a) a barefoot runner vs. (b) a shod runner when striking with the heel. Note how much bigger and sharper the initial impact transient is when not cushioned by a shoe. (From http://barefootrunning.fas.harvard.edu.)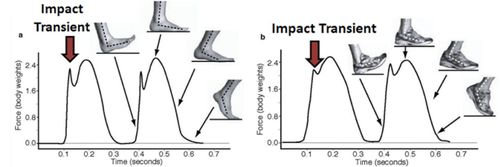
While our legs absorb huge impacts when we run, other animals use body parts in even more punishing ways to obtain food, for example, to penetrate the armor of prey. In the pantheon of amazing creatures, the stomatopods, commonly called mantis shrimp (∼400 known species), have gained fame thanks to clever research combining physical and biological perspectives. Sheila Patek's TED talk on her stomatopod research is an excellent introduction to stomatopod biology (www.ted.com/talks/sheila_patek_clocks_the_fastest_animals.html). Stomatopods have the fastest predatory strike in nature; some species use specialized forelimbs to club armored prey with great force, while other species rapidly spear fast-moving prey (Figure 3). One of the most amazing physical phenomena observed in connection with these strikes is light-emitting cavitation. Patek began studying this biologically produced cavitation when she recorded surprisingly large second peaks following the initial force generated when a stomatopod was induced to hit a force transducer (smeared with shrimp paste). She wanted to understand what was causing that second peak, and why it was happening mere milliseconds after the initial strike, a nice example of a physical measurement driving further biological inquiry. It turns out that the stomatopod limb is traveling through the water so fast and striking the prey's shell so forcefully that water is being vaporized and forming rapidly collapsing bubbles that release huge additional energy in the form of heat, pressure, and light just milliseconds after the initial strike. High-speed video captures the vapor cloud and light flash approximately 13 min into the 16-min TED talk. Cavitation is familiar as a force that destroys high-speed boat propellers, and eventually it destroys stomatopod raptorial limbs as well (physics rules), but the predators just grow a new one (biology rules, too). Analyzing the forces required and energies involved in cavitation would be an excellent biophysics exercise.
Figure 3. The raptorial forelimb of the stomatopod is used as a club in some species and as a spear in others, with high strike speeds achieved by storing mechanical energy. (Photo courtesy of Roy Caldwell, Museum of Paleontology, University of California–Berkeley.) The drawing, showing the saddle structure, is adapted from Burrows (1969) via the Arthropoda blog (http://arthropoda.wordpress.com/2010/02/09/why-stomatopods-are-awesome-i-super-strength).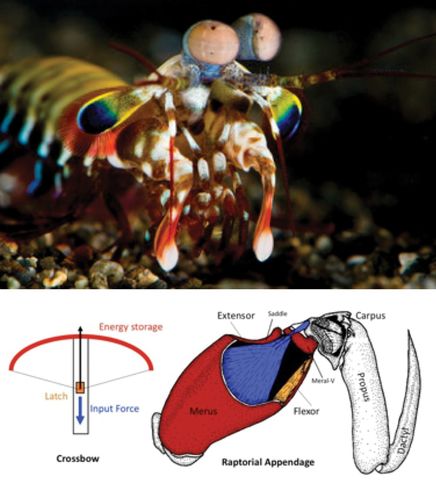
Dr. Patek has built a handsome faculty website (www.bio.umass.edu/biology/pateklab/users/sheila-patek), with images, videos, and good explanations of her research interests. Legendary invertebrate neurobiologist Malcolm Burrows was the first to analyze the biomechanics of stomatopod raptorial limbs, and Roy Caldwell at Berkeley has been a leader in probing compelling aspects of stomatopod biology. His website (www.ucmp.berkeley.edu/aquarius/signals.html) is a fascinating window into stomatopod biology, literally, because Dr. Caldwell was able to study stomatopods in their native habitats through submarine windows as part of an Aquarius project. Coral Science, an organization devoted to making coral reef research accessible, also provides a good feature on stomatopod biology (www.coralscience.org/main/articles/reef-species-4/stomatopods).
Some of the best material on the Web is in blogs these days, and a good example is a blog by Marc Srour, a young paleontologist interested mostly in arthropods (http://bioteaching.wordpress.com/2011/07/13/mantis-shrimp-crustacea-stomatopoda). Casey Dunn's lab at Brown University has a blog project called Creature Cast (http://creaturecast.org/about) that is devoted to telling zoology stories, incorporating contributor videos, mostly from graduate students and postdocs. Students might enjoy seeing the simple animated story on stomatopods (http://creaturecast.org/archives/2054-creaturecast-the-stomatopod-strike).
Sheila Patek's research has focused on a particular structure of the stomatopod raptorial limb called the saddle. The saddle is a section of shell at the strike pivot joint that is surrounded by elastic connective tissue. The saddle shape is a member of an elegant family of geometric structures called hyperbolic paraboloids (Figure 1, left). According to Patek, hyperbolic paraboloids are well known to mathematicians and are used extensively in engineering, architecture, and jewelry-making, because maximum strength is provided with minimal material, and the geometry distributes forces evenly across a light structure. (Next time you eat a Pringles potato chip, do so in the name of science education, and note the shape.) The saddle, along with other structural mechanisms, allows the stomatopod limb to store and rapidly release tremendous amounts of shell-crushing energy while using much less muscle mass than otherwise necessary.
Although the raptorial limbs of stomatopods are remarkable, perhaps the most amazing feature of stomatopods is their eyes (Figure 4), and exploring the science of vision presents one of the most engaging ways to combine physics and biology. A 5-min video from the Australian Broadcasting Corporation (www.abc.net.au/catalyst/stories/3280489.htm) is a concise primer on stomatopod vision. This video gives good explanations of why mantis shrimp can see many more colors than we can and can detect linearly and circularly polarized light. They are the only animals known to naturally detect circularly polarized light. We use circularly polarized light while sitting in a movie theater watching a 3D film through glasses with left and right polarizing lenses. The Not Rocket Science blog (http://scienceblogs.com/notrocketscience/2008/03/21/mantis-shrimps-have-a-unique-way-of-seeing), associated with National Geographic, has a good discussion of how mantis shrimp may make use of their remarkable visual capacity. Michael Bok, who also studies the stomatopod visual system, has an excellent blog on arthropods (http://arthropoda.southernfriedscience.com). He has some great underwater color photography, including some photos of larval mantis shrimp.
Figure 4. Stomatopod eyes pivot on stalks and can detect 12 color channels (as opposed to the three humans can detect), while also detecting circularly polarized light. (Photo © Roy Caldwell from the Coral Science website [www.coralscience.org].)
From childhood, we wonder at the world's color and ask “Why?” And you may be wondering why the mantis shrimp's visual system should have such remarkable capabilities. “Why” questions can be tricky. In the case of vision, research can provide deep insights into evolutionary adaptations and stimulate biologists to use physics to better understand biology. It is challenging to understand how stomatopods use their ability to see circularly polarized light for specific adaptive behavior. But from the physics perspective, we can study how the visual system extracts information from the physical world to gain some insight.
NBC news has a science portal (www.nbclearn.com/portal/site/learn/chemistry-now/chemistry-of-color) with a video focused on why flowers are colored. The transcript is available, as are a couple of lessons tied to the video. The video is a case study in how we tend to define the differences among biology, chemistry, and physics as methods of natural science inquiry. The video asserts that flowers are colored because of genes, a biological explanation. These genes make pigments that are stored in cellular structures, an explanation that mixes biology and chemistry. The video proceeds to physics, presenting the beginnings of a quantum mechanics–based explanation of how color is generated. Asking questions about flower color is an engaging way to unite science, technology, engineering, and mathematics disciplines in a single case study.
From a chemistry–physics viewpoint, there are three types of color in the world: pigment color, structural color, and luminescent color. Each type of color arises from molecules interacting with components of light in completely different ways. The online exhibit Why Things Are Colored (www.webexhibits.org/causesofcolor/7I.html) provides a good context for thinking about how biological colors are produced and perceived. Color from pigment is the most familiar. If you grind pigment, you produce finer and finer grains of the same color. Using mechanical and chemical processes, you can extract the pigment and use it to color other things. Pigment molecules absorb light of specific frequencies and reflect the light of other frequencies. The United Kingdom Chemguide website (www.chemguide.co.uk/inorganic/complexions/colour.html) dives a little deeper into the atomic-level explanation for pigmented color. Chemguide makes it clear that our understanding of color is incomplete from a quantum physics point of view. If you want more in-depth lessons, the Physics Classroom (www.physicsclassroom.com) has excellent materials, including a section on the nature of light and vision (www.physicsclassroom.com/class/light).
In contrast to color from pigment, structural color arises from light interacting with structures at a nanometer scale, the same scale as the wavelengths of light (Figure 5). If you change these nanostructures, you eliminate or alter the color. If you grind the “colored” material, you do not produce smaller bits of color, you destroy it. It could be helpful to think of structural color as a form of nanotechnology. Over time, pigments fade via chemical degradation processes, while structural color can remain amazingly vibrant long after an organism's death. The Scientist website recently published a nice feature on structural color (www.the-scientist.com//?articles.view/articleNo/34200/title/Color-from-Structure). A fascinating aspect of structural color is the diverse ways that plants, birds, and insects achieve structural color. In evolutionary convergence, beetles and birds, for example, decorate themselves blue, using entirely different macro and micro structures, with the common result being that the structures refract and reflect light such that we see blue at the surface. A good explanation of the basics of structural color can be found in a science blog affiliated with The Guardian newspaper (www.guardian.co.uk/science/punctuated-equilibrium/2007/oct/16/birds-physics). Shuichi Kinoshita and Shinya Yoshioka have published a superb review of structural color in nature (http://openwetware.org/images/8/80/Kinoshita_StructColorsNature_ChemPhysChem2005.pdf) and Minoru Taya has posted some useful curriculum materials on structural color in nature from his mechanical engineering course at the University of Washington (http://courses.washington.edu/mengr568/notes/color_in_nature.pdf). For a more mathematical treatment of structural color, have a look at a review by Michael Steindorfer and colleagues in The International Online Journal of Optics (www.opticsinfobase.org/oe/fulltext.cfm?uri=oe-20-19-21485&id=241224). In addition to blues and violets, you might be surprised to learn that white is a common structural color, since brilliant whiteness is very difficult to achieve with pigments alone. In practice, many of the colors we perceive in nature are a combination of pigment and structural color.
Figure 5. Structural color arises not from pigment, but from light being refracted and reflected while interacting with fine-scale structures. The berries are blue from structure, while the squid skin exhibits myriad colors from a combination of pigment and structure. (Berry photo courtesy of P. J Rudall. Squid photo courtesy of Grayson Hanlon from The Scientist.)
Richard Prum and others have been studying structural aspects of bird feathers for some time, examining small air pockets and organized arrays of keratin granules produce structural colors. Recently, Prum and colleagues published an article (www.pnas.org/content/early/2010/06/11/0909616107.full.pdf+html?with-ds=yes) on the structural color of butterfly wings, some of the most beautiful palettes known. Butterfly wings are covered with scales with microstructures composed of gyroids (Figure 1). Wired magazine has an online article on gyroids and structural color in butterfly wings (www.wired.com/wiredscience/2010/06/butterfly-colors). For a more technical discussion on butterfly wing gyroids and their diversity across species, see the amazing work of K. Michielsen and D. G. Stavenga (http://rsif.royalsocietypublishing.org/content/5/18/85.full). The gyroid shape is in the family of minimal surfaces, structures that are appealing if you want to make things very strong while keeping them very light. Interestingly, you could say that Alan Schoen discovered the gyroid in the 1970s while working for NASA, and you can learn more about the interesting history of this structure at his website (http://schoengeometry.com/e_tpms.html). Adam Wyhaupt publis-hed a concise article on gyroids with good graphics in +plus magazine (http://plus.maths.org/content/meet-gyroid). To have some fun playing around with gyroids and other triply minimal surfaces, visit Paul Nylander's website (www.bugman123.com/MinimalSurfaces/index.html).
One of the coolest things about how organisms produce color is that the morphology responsible for both pigment color and structural color can be detected in fossils (Figure 6). In the case of pigment, melanosome type and distribution can be used to deduce the color patterns of fossilized birds, mammals, and dinosaurs. In the case of structural color, patterns can be deduced and even seen intact when preservation is so fine that the structural color is retained in the fossil. Discovery News has a short feature on “paleocolor” research (http://news.discovery.com/animals/psychedelic-colored-insects-111115.htm). Derek Briggs at Yale University is a pioneer in paleocolor, his research stemming from his group's work on remarkably well-preserved fossils from the Cambrian and Ordovician time periods (http://people.earth.yale.edu/profile/deb47/content/research). Jakob Vinther, Briggs’ former student, has an excellent Web page on deducing color from fossils (www.jakobvinther.com/Fossil_color.html).
Figure 6. The colors of long-dead organisms can be deduced from exceptionally well-preserved fossils. A fossil feather (left) has a melanosome conformation similar to a modern feather (center). (From Jakob Vinther [www.jakobvinther.com/Fossil_color.html].) On the right, an exceptionally well-preserved fossil beetle carapace shows intact blue structural color. (From Derek Briggs [http://people.earth.yale.edu/profile/deb47/content/research].)
Interestingly, the nanometer scale of structures that accounts for structural color are smaller than the molecular scale that cellular enzymes control directly. Therefore, structural color results from self-assembly and autonomous processes that arise from the inherent properties of the constituent monomers and conditions in the extracellular milieu. Research on complex, nanoscale biological structures is in its infancy. Rather than list technical publications, I think it is useful to get students started on thinking about self-assembly principles in general. A good way to prime students to think about the rules, conditions, and constraints for self-assembly is to play the protein-folding “game” Fold-it. To get started, visit the Fold-it portal information page (http://fold.it/portal/info/about). From the simulations, which are based on actual data, students can begin to understand how the chemical and physical properties of biological molecules lead to their innate ability to self-organize into predictable shapes.
I have barely scratched the surface on the biology–physics interface in this Feature, and I hope readers are intrigued by the science education opportunities afforded by entwining physics and biology. I will close with a few comments relating to biology–physics curricula.
As many readers are aware, there is a decades-old “physics first” educational movement. The original idea was that U.S. high school students should take physics in ninth grade as a foundation for further courses in chemistry and biology. There have also been related movements and discussions at the college level. The move to put physics first in the science curriculum has had its ups and downs, mostly losing gains made in the 1990s but not completely disappearing. You can see an ongoing implementation at A TIME for Physics First (www.physicsfirstmo.org), which is affiliated with the University of Missouri–Columbia. My employer, the Howard Hughes Medical Institute, has helped to fund a project called NEXUS (http://umdberg.pbworks.com/w/page/31612279/HHMI%20Interdisciplinary%20Collaboration%20for%20Bio%20Education%20Reform) to design a course in physics specifically for biology majors. NEXUS places the physics course second, after an introductory biology course. You can view, and even participate in, this work-in-progress via various comment pages (e.g., http://umdberg.pbworks.com/w/page/49797017/Readings%20Physics%20131; scroll down to the Overview section, where you will find a nice page of physics for biologists websites at http://umdberg.pbworks.com/w/page/35656531/Physics%20for%20Biologists%20Websites).
There are many biophysics courses and departments around the world. Wikiversity has a large comprehensive biophysics course online (http://en.wikiversity.org/wiki/Biophysics#Languages:_.28en.29.2C.28fr.29.2C_.28ro.29) with some excellent graphics; text in French, English, Spanish, and German; and some sections available in numerous other languages. Phil Nelson at the University of Pennsylvania is the author of an undergraduate biophysics textbook and a leading proponent of meaningful curricular connections between biology and physics (www.physics.upenn.edu/∼pcn). You can download a version of a presentation he calls “Keeping the Physics in Biophysics” (www.physics.upenn.edu/∼pcn/1009Oxford.pdf); it is entertaining, informative, and provocative (see the quote I used in the opening paragraph of this Feature). Nelson's presentation explores what he thinks are some of the best opportunities to present interesting and important biophysics to undergraduates, primarily in the areas of molecular biophysics, genetics, and bioinformatics, but also in neural processing and imaging. As a finale, you can become inspired by nature after reading Mary Salimi's excellent review article on designs and structures that engineers have derived or copied from living organisms (www.scribd.com/doc/120688728/Biomimetics-and-bioinspiration-for-design-of-innovative-materials-and-systems).
ACKNOWLEDGMENTS
Thanks to Malcolm Campbell for helpful editorial comments.



