Th1/Th2 Cytokines: An Easy Model to Study Gene Expression in Immune Cells
Abstract
This report describes a laboratory exercise that was incorporated into a Cell Biology and Molecular Biology advanced course. The exercise was made for a class size with eight students and was designed to reinforce the understanding of basic molecular biology techniques. Students used the techniques of reverse transcription and arginase activity measurement as well as nitric oxide determination to discover whether two specific genes were expressed by cytokine-stimulated dendritic cells. The experiment served as the basis for discussing the importance of differential gene expression inside the eukaryotic cell and the importance of cytokines in the immune system.
INTRODUCTION
An elective course in immunology taught to fifth-year life science students seemed like an ideal setting to integrate molecular biology techniques that the students were learning about throughout their course work. The students were told that they had cultured dendritic cells from a retrovirally immortalized cell line. The students were then assigned the task of using reverse transcription-polymerase chain reaction (RT-PCR) to determine whether two different genes were expressed after cytokine stimulation of the cultured dendritic cells. Therefore, enzymatic assay of one of the proteins generated by the genes expressed and product determination of the other one would be performed in order to consistently probe the gene expression. During the exercise, questions were posed to the students in an open-discussion format that tested and reinforced their knowledge of general aspects of lab work, experimental design, and RT-PCR.
The Th1/Th2 Dichotomy in Immune Cells
This laboratory exercise was preceded by a classroom session that focused on the basic aspects of Th1/Th2 dichotomy. The dichotomy between Th1 and Th2 has been identified in murine CD4+ T-cells (Mosmann and Coffman, 1989), and the analysis of T-cell clones in humans has shown an analogous, although not identical, cytokine synthesis heterogeneity (Romagnani, 1994). Th1 and Th2 CD4+ T-cells differ in cytokine expression: Th1 cells produce interleukin (IL)-2 and interferon gamma (IFN-γ), whereas Th2 cells express IL-4, -5, -6, -10, and -13 (Romagnani, 1995). This cytokine heterogeneity is not restricted to CD4+ T-cells, as other cell types also contribute to the secretion of regulatory cytokines. Thus, the terms Th1- and Th2-type cytokines or cells are used to characterize the cytokine profile of different CD4 cell types.
This dichotomy also characterizes two alternative states that are often correlated with the course of a disease. The Th1 cytokines are considered proinflammatory cytokines, and they are often correlated with a gaseous messenger known to modulate specific functions of cell populations involved in the immune response. This messenger is nitric oxide (NO), a gaseous metabolite produced by the degradation of amino acid l-arginine by nitric oxide synthase (NOS). NO has been shown to be a crucial host-protective and antimicrobial effector molecule as well as a potential host-destructive mediator in diverse scenarios of immunopathology. Nevertheless, l-arginine may be metabolized by an alternative metabolic pathway. It can also be catalyzed by arginase, which converts l-arginine to l-ornithine and urea. Th2, in contrast to Th1 cytokines, often exhibits anti-inflammatory properties, and their expression has been related to the induction of arginase.
Thus, it seems that the Th1 and Th2 dichotomy generates alternative states that correlate with NOS and arginase expression, respectively (Figure 1). Previous studies demonstrated that Th1 and Th2 cytokines (Modolell et al., 1995; Corraliza et al., 1995), as well as the corresponding T-cells (Munder et al., 1998), competitively regulate the balance of l-arginine metabolism in murine macrophages and dendritic cells. Although Th1 cells and cytokines induce the inducible nitric oxide synthase (iNOS) and suppress arginase, Th2 cells and cytokines induce arginase and suppress iNOS. These studies support the idea that this competitive inhibition is based only on competition for the substrate between both enzymes. There is no evidence to support the idea of a cross-inhibition at the signal transduction level.
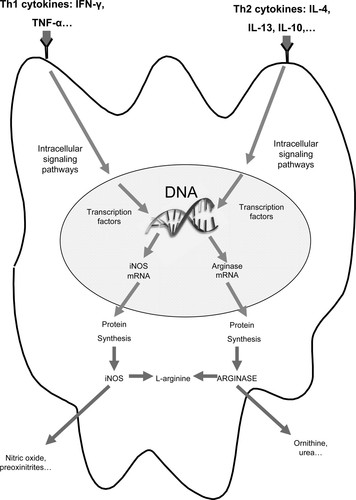
Figure 1. Th1/Th2 dichotomy regulates the iNOS/arginase expression in dendritic cells. The Th1 cytokine stimulation of dendritic cells leads to iNOS expression, but Th2 cytokine stimulation leads to arginase induction. Both enzymes iNOS and arginase share the same substrate, the amino acid l-arginine. The expression of iNOS leads to nitric oxide production, whereas the expression of arginase leads to l-ornithine and urea production.
Goals and Objectives
Once the basics of the Th1/Th2 dichotomy were discussed, the question we addressed next was whether we could differentially regulate the expression of NOS and arginase by the use of Th1 and Th2 cytokines. Obviously, the answer to the question was trivial, because a lot of previous studies demonstrated that it could be achieved (Munder et al., 1999), but we believe that its accomplishment in a laboratory exercise context would provide two interesting didactic perspectives: first, the study of the Th1/Th2 dichotomy in a practical approach and second, the study of the differential gene expression inside the eukaryotic cell, pointing out the importance of signal transduction, mRNA expression, and protein synthesis. Nevertheless, there are other important objectives that may be reached with this laboratory exercise: Experiments were fully performed by students. The theoretical basis of experimental techniques shown here were taught previously to the students, who were then able to perform in a driven way the experimental techniques themselves. In addition, the experimental design shown here was discussed previously in an open-discussion format, allowing students to be introduced to the theoretical development of a complete scientific experiment. Designing experiments on their own could be complicated for the students. They began this process by consulting the primary literature. Designing experiments from the literature poses several new experiences for students, who were more familiar with cookbook instruction in the laboratory. These include interpreting the technical language of the article (a first-time experience for many students), filling in the details for a method when limited technical information is given, resolving conflicting information from different sources, and substituting reagents or experiments when resources are limited.
Finally, the discussion of the obtained data allowed inquiry into new experimental designs that would allow development of a new set of experiments that could expand the results obtained (see below).
MATERIALS AND METHODS
Equipment Required
The following equipment was used in this exercise: biological safety cabinet for performing sterile cell culture, cell culture incubator, microcentrifuge, spectrophotometer, thermal cycler, UV transilluminator, horizontal mini-gel electrophoresis apparatus, shaker, and an electrophoresis power supply. The reagents used for cell culture, RT-PCR, arginase activity determination, NO measurement, and gel electrophoresis are as indicated below.
Cell Culture and Total RNA Isolation
The DSC2/1 is a retrovirally immortalized dendritic cell line that was generously provided by Dr. P. Ricciardi-Castagnoli (Consiglio Nazionale delle Ricerche Center of Cellular and Molecular Pharmacology, Milan, Italy). The cells were maintained in Dulbecco's modified Eagle's medium (Sigma Chemical, St. Louis, MO) supplemented with 10% heat-inactivated fetal calf serum, 2 mM l-glutamine, 60 mM 2-mercaptoethanol, 1 mM sodium pyruvate, 1× nonessential amino acids, 100 U/ml penicillin, and 100 mg/ml streptomycin (Life Technologies, Paisley, United Kingdom) in a humidified atmosphere (37°C and 10% CO2). For RNA isolation, approximately 50 × 104 cells/cm2 were seeded into 35-mm tissue culture dishes (Corning Glass Works, Corning, NY). Then, fresh medium was added, and cells were stimulated with IFN-γ (100 ng/ml; Zymed Laboratories, South San Francisco, CA) or IL-4 (10 ng/ml; R&D Systems, Abingdon, United Kingdom). Cells were cultured in the presence or absence of the cytokines tested for 6 h. Then total RNA was isolated from the cells using Tri Reagent and the protocol provided by the manufacturer (Molecular Research Center, Cincinnati, OH) and was quantified by measuring the absorbance at 260 nm. Samples were then stored at −80°C for 1 d. It is very important to avoid DNA contamination of RNA samples. PCR cannot discriminate between cDNA targets synthesized by RT and genomic DNA contamination. The method tested here is based on a one-step extraction using Tri Reagent according to the manufacturer's protocol. This method is a modification of the classical multisetup method of RNA extraction by the use of guanidinium thiocyanate/acid phenol:chloroform extraction and has been widely tested. In any case, a control of genomic DNA contamination should be carried out, as will be shown below.
RT-PCR
The technique of RT-PCR was performed the next day using 3 ng of total RNA from the D2SC/1 dendritic cells. RT was performed as shown in Table 1. The reaction was carried out, resulting in a final volume of 30 μl containing 0.4 mM of each dNTP and 150 ng pd(N)6 (all from Pharmacia, Freiburg, Germany), 200 U Moloney murine leukemia virus reverse transcriptase, 1 mM DTT (both from Invitrogen, Carlsbad, CA), 50 mM Tris-HCl (pH 8.3), 5 mM MgCl2, and 62.5 mM KCl and 40 U RNase-OUT (from Invitrogen). The use of RNase-OUT RNasin reduces the activity of ribonuclease (RNase) A-type enzymes in a variety of organisms. Samples were then stored at −40°C (−20°C can also be used). Table 1 shows the composition of each mixture.
| 1. Prepare the following RNA/primer mixture in each tube: | |
| Total RNA | 3 ng |
| Random hexamers (50 ng/μl) | 3 μl |
| 8 mM dNTP mix | 1 μl |
| DEPC H2O | to 8 μl |
| 2. Incubate the samples at 65°C for 5 min and then on ice for at least 1 min. | |
| 3. Prepare reaction master mixture. For each reaction | |
| 10× RT buffer (500 mM Tris-HCl, pH 8.3, 625 mM KCl) | 2 μl |
| 25 mM MgCl2 | 4 μl |
| 0.625 M KCl | 2 μl |
| 10 mM DTT | 2 μl |
| RNase-OUT | 1 μl |
| 4. Add the reaction mixture to the RNA/primer mixture, mix briefly, and then place at room temperature for 2 min. | |
| 5. Add 1 μl (200 U) of Moloney murine leukemia virus reverse transcriptase to each tube, mix, and incubate at 25°C for 10 min. | |
| 6. Incubate the tubes at 37°C for 30 min, heat-inactivate at 70°C for 15 min, and then chill on ice. | |
| 7. Store the first-strand cDNA at −20°C until use for PCR. | |
The following day a total of 1 μl of the resulting cDNA (adjusted to a concentration of 50 ng/ml input RNA) was then amplified by PCR in a 50-μl reaction mixture containing 0.2 mM of each dNTP, 1 mM DTT, 200 nM of each primer, 0.6 U Taq polymerase (HT Biotechnology, Cambridge, United Kingdom), 10 mM Tris-HCl (pH 9.0), 1.5 mM MgCl2, 50 mM KCl, 0.01% (wt/vol) gelatin, and 0.1% Triton X-100. PCR amplification was performed in a DNA thermal cycler (Perkin Elmer-Cetus, Norwalk, CT) for 35 cycles after an initial denaturation step for 5 min at 95°C with the following parameters: 20 s at 95°C (denaturation), 20 s at 56°C (annealing), and 30 s at 72°C (extension), and a final extension of 10 min at 72°C. “No template” sample consisted of molecular biology grade water instead of RNA, and the “minus reverse transcriptase” sample contained 1 μl of cDNA from unstimulated cells where used as controls (see below). Table 2 is a brief guide on how to carry out the PCR.
| 1. Dilute the samples in molecular biology quality water so that you get a concentration of 50 ng/ml. For example, if you took 20 μg of RNA at the beginning, dilute your sample up to 400 μl. | ||||
| 2. Prepare the following PCR reaction mixture for each tube: | ||||
| H2O | 30 μl | |||
| cDNA (50 ng/μl) | 1 μl | |||
| PCR buffer (10×; 10 mM DTT, 100 mM Tris- HCl, pH 9.0, 15 mM MgCl2, 500 mM KCl, 0.1% (wt/vol) gelatin, and 1% Triton X-100) | 5 μl | |||
| 1 mM dNTP mix | 10 μl | |||
| Sense primer (5 μM) | 2 μl | |||
| Antisense primer (5 μM) | 2 μl | |||
| Taq polymerase (15 U/μl) | 0.04 μl | |||
The sequences of the primers used are as follows: arginase I sense primer, 5′-CAGAAGAATGGAAGAGTCAG-3′ and arginase I antisense primer, 5′-CAGATATGCAGGGAGTCACC-3′ generating a 250-base pair PCR product that spans two introns of the arginase I gene; and iNOS sense primer, 5′-TCACGTTTGGGTCTTGTTCAC-3′ and iNOS antisense primer, 5′-AAATCCTACCAAAGTGACCTG-3′ generating a 180-base pair PCR product that spans two introns of the iNOS gene. The primers for the housekeeping gene β-actin amplify a 348-base pair PCR product, and the sequences are as follows: β-actin sense primer, 5′-TGGAATCCTGTGGCATCCATGAAAC-3′ and antisense primer 5′-TAAAAACGCAGCTCAGTAACAGTCCG-3′.
Gel Electrophoresis
The gel was loaded at the beginning of the class on the fourth day. Samples were then electrophoresed at 95 V for approximately 30–45 min. The electrophoresis was carried in 1× TBE buffer (Tris-borate EDTA; supplied by Pronadisa, Madrid, Spain). The PCR products were run on a 1.5% agarose gel and stained with ethidium bromide (0.5 mg/ml). When handling ethidium bromide, care should be taken and gloves must be used. The DNA was visualized using an UV transilluminator (λ = 365 nm), and image captures were taken for the students using a digital camera. While the gel was running, the biochemical basis of RT-PCR and the use of controls in RT-PCR were discussed. Other topics that were discussed included the importance of differential gene expression and its mechanisms in eukaryotic cells.
Arginase Activity and NO Determination
The presence of arginase and NOS after gene expression was addressed by the determination of arginase activity and NO production, respectively.
Arginase activity was measured in cell lysates with slight modifications as previously described (Corraliza et al., 1994). Briefly, cells were lysed after a 24-h incubation in the presence and absence of 100 ng/ml IFN-γ and 10 ng/ml IL-4. The lysis buffer consisted of 100 μl of 0.1% Triton X-100. Lysis was performed for 30 min on a shaker, and then 100 μl of 25 mM Tris-HCl, pH 7.4, was added. Arginase needs Mn2+ as a cofactor; thus 10 μl of 10 mM MnCl2 was added to each tube. Finally, the enzyme was activated by heating for 10 min at 56°C. Arginase reaction was conducted by incubating the lysate with 100 μl of 0.5 M l-arginine (pH 9.7) at 37°C for 15 min (samples induced with IL-4) or 120 min (samples induced with IFN-γ and controls). The reaction was stopped with 900 μl of H2SO4 (96%)/H3PO4 (85%)/H2O (1/3/7, vol/vol/vol). This solution is very acidic, and it should be used very carefully. All manipulation should be done wearing appropriate clothes, gloves, and glasses. Urea is generated as the final product of this reaction. The urea concentration was measured at 540 nm after addition of 40 μl α-isonitrosopropiophenone (3% dissolved in 100% ethanol), followed by heating at 95°C for 30 min. After heating, tubes may open unexpectedly, so they should be taken from the heater carefully, always with the use of gloves. Tubes were then cooled for 30 min at 4°C. Because the generated color (close to violet) is light sensitive, the tubes should be handled in the dark. One unit enzyme activity is defined as the amount of enzyme that catalyzes the formation of 1 mmol of urea per min. Altogether this part of the exercise can last up to 5 h, so the schedule should be planned carefully. Some indications on how to facilitate scheduling these experiments are described below.
Moreover, NO was measured as nitrite using the Griess reagent (Green et al., 1982). Cell culture supernatant was mixed with 100 μl of 1% sulfanilamide, 0.1% N-(1-naphthyl) ethylendiamine dihydrochloride, and 2.5% H3PO4. Absorbance was measured at 540 nm in a microplate reader (Molecular Devices, Ismaning, Germany).
Cost Considerations
Although the use of experimental kits may seem to limit student understanding of the science underlying the techniques being utilized, if used properly they can be useful and cost-effective tools. Most of the suppliers of molecular biology reagents and kits now have extensive Web sites and technical literature outlining the science behind their kits. We recommend that authors use the kits to extract total RNA and to perform RT-PCR. The kits can be purchased for approximately $35 per student per laboratory exercise (including general reactives), assuming eight students per course. Cultured cell reactives (including cytokines) and media will increase the cost by approximately $30 per student. The major costs associated with this project are the thermalcycler and the flow chamber needed to culture the cells.
Guidelines for the Programming of the Laboratory Exercise
This project can be adapted depending on the number of hours students spend in the laboratory each day. The experimental activity should be conveniently scheduled because of the high amount of “dead time” that may be generated between the incubation periods. Anyway, this dead time is an exceptional opportunity to discuss the experiments with the students.
We recommend that authors develop the experimental activity according to the following guidelines.
Day 1.
Classroom session dedicated to discussing particular aspects of the experiments. Then the cells were seeded in 35-mm dishes.
Day 2.
In the early morning, culture medium was removed, and fresh medium was added then. Cells were stimulated with 100 ng/ml IFN-γ and 10 ng/ml IL-4 for 6 h. Cells without induction were used as controls. Another set of cells with identical conditions was induced. These cells would be incubated for 24 h for arginase activity and NO production tests. After 6 h of incubation, total RNA was extracted and frozen at −80°C. It is possible to freeze RNA at −40°C, but its stability is much lower because of RNase activity. A short aliquot of RNA from each sample was measured in order to quantify it.
Day 3.
After 24 h of incubation, arginase activity was measured. To optimize the time, supernatants for NO determination may be stored at −20°C. Arginase activity determination has a dead time of 120 min where RNA samples may be defrosted and RT could be performed. After the RT was performed, the cDNA could be stored at −20°C.
Day 4.
PCR was performed. While the PCR program was running, the NO determination could be done. An agarose gel electrophoresis must be done to test the results. While it is running is a good moment to discuss final aspects and to discuss the potential difficulties found (see below).
Day 5.
Final exposition and discussion of results of each student.
Assessment of Learning Objectives
Assessment of our course takes the form of how effectively we meet our intended student learning outcomes. We have implemented assessment tools to measure the effectiveness of our student learning objectives. Our student learning objectives have been developed based on Bloom's hierarchical taxonomy of the cognitive domain (Bloom et al., 1956). Using this structure, we have developed a classification of our learning objectives and expected outcomes (Table 3). To reinforce student learning, formative assessment exercises were provided in association with lectures and tutorials. Over the past 3 yr, 72 students have participated in this evaluation by means of this online assessment. The assessment tool measures every expected objective. The assessment items that were developed examined both factual recall and higher-order thinking, including integration of knowledge and problem-solving ability.
| Goal and objective | Expected outcome | Level of knowing |
|---|---|---|
| Provide knowledge content across the full range of biology | Ability to retrieve information from databases | 1. Knowledge |
| 2. Comprehension | ||
| Generate understanding of concepts in biology | Ability to communicate knowledge and concepts both in writing and orally | 1. Knowledge |
| 2. Comprehension | ||
| 3. Application | ||
| 5. Synthesis | ||
| Understand and use scientific methodology | Ability to design laboratory experiments | 1. Knowledge |
| 2. Comprehension | ||
| 3. Application | ||
| Ability to perform good laboratory practice | 2. Comprehension | |
| 3. Application | ||
| 4. Analysis | ||
| Promote familiarity with a range of methods and techniques relevant to application of the biological sciences | 1. Knowledge | |
| 2. Comprehension | ||
| 3. Application | ||
| 5. Synthesis | ||
| Foster critical thinking | Ability to determine the veracity and value of published information | 6. Evaluation |
| Propose ways to advance knowledge in biology | Foster ownership of ideas, research, concepts, knowledge, and effort | 6. Evaluation |
Students and teachers need regular and constant feedback to better assess student progress inside the laboratory experience. Consider giving short quizzes on individual topics to assess the level of mastery the students have achieved. We recommend developing a short quiz of three or four questions that incorporates a range of these levels (Table 4), which will help teachers assess each student's mastery of the topics covered. In addition, students can use this information to pinpoint their areas of weakness.
| Bloom's taxonomy level | Sample questions |
|---|---|
| Knowledge | What is RT-PCR? |
| List the agonists that you could use to induce NOS activity under experimental conditions. | |
| Comprehension | What is the difference between Th1 and Th2 cytokines? |
| Can you provide a definition for Th1/Th2 dichotomy? | |
| Application | Write one example of RT-PCR utility. |
| Could the NOS expression have happened under Th2 cytokine stimulation of dendritic cells? | |
| Analysis | Identify reasons for using cytokine-induced cells as a model for determining gene expression. |
| Compare RT-PCR and PCR, and explain the differences between each. | |
| Synthesis | What would happen if arginase and iNOS were expressed at the same time in the same cell? |
| Evaluation | What changes to the designed experiment would you recommend? |
Related to the methodology used, students submitted their answers electronically and received instant feedback on their performance. Students were therefore alerted to deficiencies in their knowledge and understanding while actively engaged in the learning. The assessment tasks were devised in-house using the WebCT package and consisted of a mixture of multiple-choice questions and “fill-in-the-box”-type exercises. Short-answer questions were also included, but unlike the other question types, these were not computer marked. Selected questions are shown in Table 5. There were no limits placed on the number of times that a student could attempt each formative assessment exercise, although WebCT allows such restrictions to be imposed if required. When analyzing student results, we looked at the mark that students achieved on their first attempt at a test. However, WebCT also allows the highest mark for a test to be recorded instead. After the completion of this set of exercises, students were given the assignment to write a journal-style report. This report should include a histogram representation of both arginase activity versus the incubation condition and nitrite concentration versus the incubation condition. In addition, students were asked to discuss the potential use of inhibitors of iNOS and arginase in order to determine if their respective inhibition causes and increases the activity of the reciprocal enzyme.
| Selected test questions |
| 1. DNA gel electrophoresis is similar to SDS-PAGE of proteins because in both cases a plot of log (molecular weight) vs. distance migrated is linear. |
| both techniques rely on a constant charge-to-mass ratio. |
| both techniques utilize the sieving properties of gels. |
| molecules migrate to the anode in both cases. |
| All of the above are correct. |
| 2. Which of the following statements is incorrect about most Taq-polymerases used in PCR? |
| They require a primer. |
| They synthesize in the 5′ to 3′ direction. |
| They require a template. |
| They synthesize in the 3′ to 5′ direction. |
| They have 3′-5′ exonuclease activity. |
| 3. Which of the following statements about primers used in PCR is not true? |
| They bracket the region of interest. |
| They both complement the DNA of the same strand of the template. |
| They attach at the 3′ end of the template strand. |
| The base pair sequence is known. |
| Selected short answer question: |
| What role do each of the following play in RT-PCR: dNTPs, Taq polymerase, primers, and retrotranscriptase? |
| Selected fill-in-the-blank question: |
| Both the Th1 and Th2 subsets are produced from a noncommitted population of precursor __.a |
| The Th1/Th2 concept rests largely on a dichotomy of __ profiles.b |
RESULTS
Each student is responsible for his/her own set of experiments. The results shown here belong to a student who was able to accomplish the exercise with minor inconvenience. The results of the RT-PCR are shown in Figure 2. The arginase I product was amplified from the D2SC/1 cells stimulated with IL-4, whereas the iNOS product was amplified under IFN-γ stimulation. No products were amplified when reverse transcriptase was excluded from the reaction mixture (minus reverse transcriptase control), when no cytokines were used, or when water was used instead of RNA (no template control). Expression of the housekeeping gene actin was confirmed for both the arginase and iNOS samples.
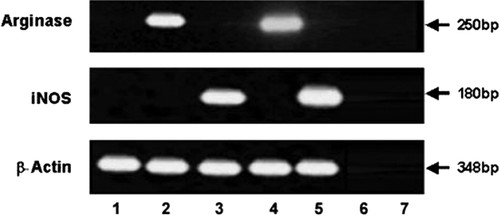
Figure 2. Cytokine-mediated induction of arginase I mRNA and iNOS mRNA in dendritic cells. D2SC/1 cells (1 × 106) were incubated with the indicated cytokines (10 ng/ml IL-4 and 100 ng/ml IFN-γ) for 6 h. Then RNA was extracted and cDNA was prepared. A total of 1 μl DNA (corresponding to 50 ng RNA) was amplified by PCR with primers specific for arginase I and iNOS. To control for comparable amount of cDNA, a β-actin mRNA was also amplified with 0.01 μl input cDNA. Lines: (1) no cytokine added; (2) IL-4 10 ng/ml; (3) IFN-γ 100 ng/ml; (4) arginase positive control; (5) bone marrow macrophages induced with IFN-γ (iNOS positive control); (6) minus reverse transcriptase control; and (7) no template control.
Because mRNA expression was detected, protein activity was addressed. The results of the arginase activity and NO production are shown in Figure 3.
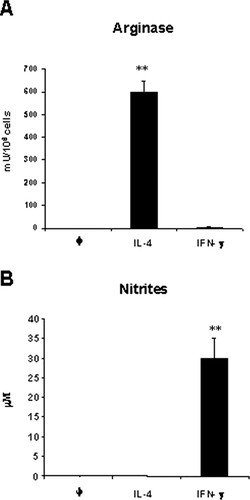
Figure 3. Induction of arginase and nitric oxide in D2SC/1 dendritic cells. D2SC/1 cells (1 × 106) were incubated with the indicated cytokines IL-4 (10 ng/ml) and IFN-γ (100 ng/ml). After 24 h, arginase activity (A) and NO release (B) were determined as described in Materials and Methods. Nitrite or arginase activity was not detectable in unstimulated cells (φ). ** Significantly different (p < 0.01) from untreated cells.
Arginase I activity was clearly augmented after IL-4 induction. On the other hand, nitrites due to NO production were detected under IFN-γ incubation conditions. No activity was detected when D2SC/1 cells were unstimulated.
DISCUSSION
Potential Difficulties in the Development of the Exercise
Students faced two major challenges in setting up and carrying out their experiments. The first of these was discovering how much time it takes to do all of one's own preparation. Students must be helped to gain a better ability to estimate the time required to do experiments.
The second major challenge students faced was troubleshooting experiments that did not work. There could be simple problems for an experienced researcher (i.e., a reactive that did not dissolve), the solutions of which might not be immediately obvious to most students. The instructor's role in these situations is to guide the student to a solution, usually through probing questions, rather than to “fix the problem.” In this laboratory exercise students were also encouraged to troubleshoot each other's experiments. This was done both informally and more formally in the discussion session.
Anyway there are major aspects that must be carefully carried out. Some steps of the experimental procedure are really critical, and they could seriously compromise the overall result of the experiment. Basically, there are three major points that must be focused on: 1) avoid contamination of cultured cells, 2) avoid RNA contamination with RNases, and 3) avoid DNA contamination in RT-PCR.
Cell Culture Troubleshooting
Cell culture is filled with variables that can make it difficult to determine the cause of problems. Narrowing a problem down to the one material or one critical procedure can be a daunting task. However, problems usually can be identified by carefully examining the symptoms and meticulously retracing each step in the culture process.
Almost every problem encountered in cell culture can be identified as one of the following:
The cells are growing poorly or not at all.
The cells have an abnormal morphology.
The materials used were inappropriate, compromised, or contaminated.
The cultures were exposed to the wrong type of environment.
The cells were exposed to toxic conditions, contamination, or nutritional deficiency.
The cell culture technique was not correct for the cell type.
If the parameters indicated above are controlled, there could be problems with toxic reagents (maybe the wrong concentration or the wrong reagent) or contamination. Microbial contamination is the most common problem we have found, and it is always associated with a culture technique not correctly developed. Microbial contamination comes in many forms including Gram-positive and -negative bacteria, mycoplasma, viruses, molds, and yeast. Indicators of contamination include turbid culture media, changed growth rates, abnormally high pH, poor attachment, multinucleated cells, grainy cellular appearance, vacuolization, inclusion bodies, and cell lysis.
Nutritional conditions of the culture must be controlled; do not forget that these cells (as well as others that could be used) need supplementation with l-glutamine.
Finally, if the cell culture technique does not produce a contamination of the culture, there could be other problems that must be addressed. Aggregation and coupling of the culture are the major problems, due to cell damage during subculturing or not fully resuspending before seeding, respectively.
Avoiding RNA Contamination with RNases
After contamination of the cultures, the major problem found was contamination of extracted RNA with RNase. In the RNA extraction step, it is really important to fully follow the instructions of the manufacturer. RNases are very stable enzymes responsible for RNA hydrolysis. RNases can be temporarily denatured by extreme conditions, but RNases readily renature. Therefore, RNases can easily survive autoclaving and other standard methods of protein inactivation. Because RNases are present in the oils of skin, gloves should be worn at all times. Gloves will also protect the researcher from contact with the solutions. It is recommended that protective eyewear be worn at all times. To avoid RNase contamination, keep lids on tubes until ready to use; work with disposable, individually wrapped, sterile plasticware; use only sterile, new pipette tips (handled with gloves only) and microcentrifuge tubes; and avoid equipment and areas of the laboratory that have contact with RNases (e.g., centrifuge tubes used for DNA preparation that may have contained concentrated RNase mixture or gel boxes that have been used for RNase-treated DNA samples).
Avoiding DNA Contamination in RT-PCR
Although DNA contamination is easily detected by performing a “no-RT” control, there is no easy remedy. PCR cannot discriminate between cDNA targets synthesized by RT and genomic DNA. As discussed below, it is important to design good controls to avoid this situation. A contamination of genomic DNA could easily lead to a false-positive result. Although there are methods to remove DNA contamination from RNA samples, this is not the objective of this laboratory exercise. If you have a result in the “no-RT” control, just reject the sample.
Introduction to RT-PCR.
The biochemical basis of RT-PCR (Jones, 2002; DNA Learning Center, 2005) was discussed with the students, specifically, the preparation of first-strand cDNA from RNA by the use of random hexamers. Alternative methods of priming the RNA to generate first-strand cDNA, such as the use of oligo(dT) or random hexamers, were also mentioned. The necessity of the enzyme reverse transcriptase and nucleotides (dNTPs) in the reverse transcription mixture was explained. Once the first-strand cDNA is synthesized, the students were told how exponential amplification for the target sequence located between the designated primers can be performed by using DNA polymerase and sense (forward) and antisense (reverse) primers for arginase I, iNOS, and actin. The importance of magnesium in the reaction mixture for both primer annealing and polymerase activity was also pointed out. An important point that was emphasized was that the main steps of PCR include repeated cycles of DNA denaturation, primer annealing, and extension from the primers mediated by a heat-stable DNA polymerase.
The Importance of Positive and Negative Controls in RT-PCR.
Because of the considerable amplification potential of RT-PCR, the use of both negative and positive controls and the importance of such controls in quality assurance was discussed (Lion, 1996, 2001). The use of a “no template” or water control is an important means of determining if your reagents are contaminated with the cDNA that is being amplified, and the use of a “minus reverse transcriptase” control is a useful way of determining if your reverse transcriptase is contaminated with the cDNA that is being amplified. The use of PCR primers that span an intron is one way to determine if your RNA sample is contaminated with genomic DNA. Because the primers for arginase and iNOS that were utilized span two introns, the amplification of products with the predicted size indicates that the generated PCR product did not result from PCR amplification of DNA contamination of the RNA sample (Figure 2). Alternatively, incubating experimental samples with the enzymes DNase or RNase before RT-PCR is another way to determine if your product is being amplified from RNA or DNA templates. A positive control for RNA integrity/degradation includes performing RT-PCR for an abundant mRNA species such as the housekeeping gene β-actin.
Specificity of PCR Primers for Arginase, iNOS, and Actin and Identifying a PCR Product.
As previously indicated, one of the critical aspects for RT-PCR is the primer choice with respect to minimizing the problems associated with DNA contamination. Primers must be designed spanning at least one intron of the genomic sequence. The resulting PCR product from genomic contamination will be larger in size than the product generated from the cDNA. In fact, primers can be designed to span a sufficiently large genomic fragment, so that amplification from contaminating DNA may not be possible. In genes for which the genomic sequence has been published, the positions of the splice junctions can be found by retrieving the sequence from the GenBank database (GenBank, 2005). If the intron–exon structure is unknown, primers can be synthesized in different regions of the cDNA sequence, and different combinations can be tried on both cDNA and genomic DNA. It should be possible to choose a primer combination that yields either no product (additional intron sequences render the target taken for efficient PCR) or an easily distinguishable product when amplifying from genomic DNA. An additional problem is that pseudogenes exist in the mammalian genome for many genes, including the most commonly used internal controls (β-actin, GAPDH, and cyclophilin). These sequences, arising from integration of a reverse transcription product into the genome, do not have introns. Thus, the size of a PCR product amplified from a pseudogene may be identical to that produced from a cDNA copy.
As previously described, iNOS primers and arginase primers span two introns, allowing the detection of DNA contamination. The specificity of the primers for rat arginase I, rat iNOS, and rat actin were demonstrated to the students by performing a nucleotide BLAST search on the arginase I, iNOS, and actin primer sequences using the Internet (GenBank, 2005). The PCR products were identified by sending them to a DNA-sequencing service.
Alternative methods for verifying the identity of the PCR products were discussed, including restriction enzyme analysis, Southern blotting, and nested PCR.
Alternative Experiments.
The results obtained indicated that IFN-γ was able to induce iNOS expression, and IL-4 was able to induce arginase expression. This is trivial and its pedagogical contribution is circumscribed, as was previously indicated. Nevertheless, there are alternative/future experiments that can contribute to develop new perspectives. Some alternatives were discussed with the students, but they were not explored at this time. Some of these proposals are widely described in the literature, so they may be explored in a context of a “self-design” experiment with the students. Alternative experiments that can be carried out in the same context are as shown below.
The cross-inhibition could easily be tested by the use of Th1/Th2 cytokine cocktails. Simultaneously, induction of dendritic cells with IL-4 and IFN-γ at the concentrations tested in this laboratory exercise will show the inhibition of arginase and NOS activity due to the competition for the substrate l-arginine.
The use of arginase and iNOS inhibitors such as l-hydroxyarginine or NG-nitro-l-arginine-methyl ester (l-NAME) will result in an augmentation of the activity of the noninhibited enzyme. Thus, the use of l-NAME, a widely used iNOS inhibitor, will result in an increase of arginase activity under IL-4 induction, and conversely, the use of l-hydroxyarginine under IFN-γ induction will result in an increase of NO production.
Other alternative pathways at the signal transduction level may be proved, all of them in a strict scientific field because they have not been sufficiently tested. The inhibition of the IL-4 signal transduction pathway or the inhibition at different levels of the IFN-γ signaling pathway may be tested with unpredictable results.
The use of Th2 cytokine combinations such as IL-4 and -10 or the use of Th1 cytokines such as IFN-γ and TNF-α will increase spectacularly the arginase activity or the NO production due to a synergism effect in their combined action, as previously described.
Student Response.
To further assess our objectives, surveys were administered to the students to determine their goals and aspirations. Selected responses are shown in Table 6. In general, students reported that it was difficult, but very instructive (Q ≠ 1–2). Overall, most students felt they had a much better understanding of “real world” life in the laboratory after the course. Students reported that they derived a lot of satisfaction from the self-designed section and preferred it to directed instruction (Q ≠ 4–5). The discussion session generated a number of questions on RT-PCR, signal transduction, and gene expression. Students explored the work in the laboratory and said they felt like real scientists; they discussed their experiments and techniques and saw how their data led to the formation of molecular biology key concepts. Emphasizing the experimental basis of the laboratory exercise, the students were motivated and provided with the necessary tools to read scientific journals. A class size of 5–10 students seems to be ideal for carrying out the laboratory exercise; nevertheless, the best group size depends on the resources available in the laboratory. In our laboratory, the best size was eight students working in pairs. One of the main characteristics of this exercise is that it allows a real presentation of what the students learn in the theoretical sessions. Students reported that the combination of experimental learning with self-directed experiments plus theoretical sessions was well done and fitted together (Q ≠ 3).
This exercise could also be expanded to include confirmation of the enzyme expression by Western blotting and, as previously indicated, a complete set of alternative experiments could be developed if necessary. This laboratory exercise illustrates how topics such as molecular biology, immunology, and biochemistry can be integrated within the context of a pedagogically relevant scenario. A first-hand knowledge of molecular biology techniques becomes increasingly important to the science students as these techniques are being utilized more and more in science and medicine.
| SA | A | N | D | SD | |
|---|---|---|---|---|---|
| 1. This course has improved my overall performance in the laboratory. | 60 | 20 | 20 | 0 | 0 |
| 2. This course has improved my basic understanding of experimental biochemistry and molecular biology. | 70 | 30 | 0 | 0 | 0 |
| 3. In this course the class activity, labs, reading, and assignments fitted together. | 30 | 40 | 30 | 0 | 0 |
| 4. I derived a lot of satisfaction from setting up my own project. | 50 | 40 | 10 | 0 | 0 |
| 5. I prefer to do directed experiments, rather than an independent project. | 0 | 0 | 40 | 50 | 10 |
ACKNOWLEDGMENTS
We thank Dr. Nicola Vahsen for her assistance and Drs. M. Modolell and M. Munder for helpful discussions. R.A.G.-P. was supported by a reincorporation fellowship from Junta de Extremadura (Spain). This work was supported by Grants 2PR04B002 from Junta de Extremadura and PI040828 from FIS (ISCIII, Spanish Health Ministry).



