Biotechnology Apprenticeship for Secondary-Level Students: Teaching Advanced Cell Culture Techniques for Research
Abstract
The purpose of this article is to discuss small-group apprenticeships (SGAs) as a method to instruct cell culture techniques to high school participants. The study aimed to teach cell culture practices and to introduce advanced imaging techniques to solve various biomedical engineering problems. Participants designed and completed experiments using both flow cytometry and laser scanning cytometry during the 1-month summer apprenticeship. In addition to effectively and efficiently teaching cell biology laboratory techniques, this course design provided an opportunity for research training, career exploration, and mentoring. Students participated in active research projects, working with a skilled interdisciplinary team of researchers in a large research institution with access to state-of-the-art instrumentation. The instructors, composed of graduate students, laboratory managers, and principal investigators, worked well together to present a real and worthwhile research experience. The students enjoyed learning cell culture techniques while contributing to active research projects. The institution's researchers were equally enthusiastic to instruct and serve as mentors. In this article, we clarify and illuminate the value of small-group laboratory apprenticeships to the institution and the students by presenting the results and experiences of seven middle and high school participants and their instructors.
INTRODUCTION
Biotechnology is an enticing subject for secondary-level students. It is interdisciplinary in nature, like many contemporary research problems, drawing upon research in biology, chemistry, and physics. Furthermore, research in biotechnology and biomedical engineering is expanding out of interest and necessity, with applications ranging from biosensors (Martin and Mitchell, 1998; Whitesides et al., 2001), biomaterials (Morse, 1999; Tsukruk, 2001; von Recum, 1999; von Recum and van Kooten, 1995), tissue engineering (Ramila and Vallet-Regi, 2001; Takayama et al., 1999), biomimicry (Lee, 1998; Sarikaya and Askay, 1994), nanotechnology (Smalley, 2001; Sone et al., 1999; Soong et al., 1999; Zhang et al., 1999), and advanced therapeutic and diagnostic techniques (Baker et al., 2001; Desai et al., 1999; Fodor, 1997; Kricka, 1998; Lakshmi and Martin, 1997; Schneider et al., 2001; Taton et al., 2000; Wilding et al., 1998). Researchers from many traditional biological and engineering fields are now working in this hybrid field; however, it is an appropriate time to introduce the next generation of would-be scientists to the problems and techniques relevant to this exploding, high-tech discipline and possibly spark their interest before they begin their college careers. For this purpose, a summer apprenticeship was organized and evaluated as a means 1) to teach cell culture techniques, 2) to facilitate an educational continuum between high school and college-level biology by providing practical research experience using high-tech equipment, and 3) to provide science mentorship by working alongside and contributing to the projects of expert scientists. The program satisfied high school students seeking individual summer research opportunities, and the formal small group apprenticeship (SGA) organization effectively met the interests of the researchers and the institution. Once established, the program piqued the interest of students of pre-high school age desiring to experience and explore biomedical engineering research.
This biotechnology SGA was organized and evaluated as a novel and efficient method to teach students cell culture practices for biomedical engineering, and to introduce them to graduate-level research. During this study, students experienced biomedical research hands-on in an active laboratory setting, advised by researchers from relevant but diverse disciplines and levels of training. The group apprenticeship style of teaching focused on completing sections of active research projects to be cost effective, and to provide students a realistic introduction to cellular biology and biomedical engineering research not typically introduced in a high school curriculum. The program was informal and flexible to encourage interaction with researchers, with adequate problem-solving opportunities and time to practice laboratory skills to evaluate technique performance. The title of the biomedical apprenticeship presented in this case study was “An Introduction to Advanced Cell Culture Techniques for Biotechnology: Applications to Test Biomaterial Cytotoxicity and Measure Controlled Cell Growth on Patterned Substrates.”
MATERIALS AND METHODS
The Setting and Facilities
The study environment was comprised of a biomedical engineering biochemistry and cell culture laboratory, two fluorescent imaging laboratories, and a computer laboratory. The host institution was a large Midwest academic research institution equipped with state-of-the-art imaging equipment and cell culture facilities. The instructors and participants had access to these facilities including cell culture facilities, a flow cytometry and laser scanning cytometry laboratory, and related equipment. Students assembled twice a week for 5 weeks for a total of 11 sessions, equivalent to 44 contact hours. The course was developed by a team consisting of the director of the Biomedical Engineering Center who first inspired the students during a public talk, a principal investigator in biomedical engineering with a research interest in biomedical applications to cardiovascular disease, a senior researcher with experience teaching intermediate-level laboratory science, the research manager of the Core Imaging Facility and Flow Cytometry Laboratory, and the director of a local community science program for talented high school learners.
Research Design
A small-group research apprenticeship in biomedical engineering was created and evaluated as an effective method to instruct cell culture techniques and to mentor high school students using actual research problems during the case study. Laboratory classroom observations, informal and formal interviews, and a poster presentation analysis were used to shape our perception and evaluation of the SGA study.
Designed to introduce students to contemporary laboratory skills related to cell culture, participants completed two separate but interrelated projects pertaining to larger projects in our lab. The first project was to quantitate the cytotoxic effects of adhesive test materials using flow cytometry, and the second project was to examine controlled-cell growth on micropatterned silicon substrates using laser scanning cytometry. The two projects required familiarity with culturing two different cell lines, a human monocytic cell line, THP-1, and an adherent mouse aortic endothelial cell line. Furthermore, addressing these two problems required that the students fluorescently label and image cells, and study mechanisms of cell death. The flow cytometer and laser scanning cytometer are state of the art and only accessible at a large research institution.
The specific aim of the course was to teach the participants modern techniques in cell culture. In the end, participants were trained basic cell culture techniques using both adherent and nonadherent cell lines and fluorescent imaging techniques not available in standard high school curricula. The participants were directed by undergraduate-, graduate-, and senior-level researchers working in related fields. The students were involved throughout the apprenticeship selecting topics, performing the experiments, and analyzing data. Students assisted in designing each experiment and learned the techniques while solving specific problems and yielding potentially useful data.
The specific aims for the SGA in biotechnology are outlined in Table 1. The course syllabus outlined in Table 2 was produced from list of specific aims and was implemented during the SGA.
| 1. Introduce field of biotechnology. One hour lectures given by the director and principal investigators to provide an exciting introduction to the course, defining our institutes' niche in the broad spectrum of medical-device technology, biomedical engineering and applications that we are most interested in, including implantable, angiogenesis-modulating devices. |
| 2. Define purpose of the SGA (to learn cell culture and imaging techniques to contribute to an active laboratory project) and purpose of biomedical engineering research (basic research and developing new materials, devices, and techniques for biomedicine). |
| 3. Define specific problems to solve, e.g., for problem-based learning (to test adhesive material biocompatibility and to test ability to control cell growth on silicon substrates). |
| 4. Perform literature search to familiarize students with field and techniques. Start with computer search of material safety data sheets, related scientific papers, and product data sheets accompanying assay kits. |
| 5. Complete laboratory safety course highlighting practices in laboratory research and standard operating procedures for particular equipment of interest. |
| 6. Perform experiments, evaluate results to assess success in exercising techniques and repeat experiment to improve technique, understand fully the experimental protocol, and obtain statistically substantiated results. |
| 7. Communicate results to classmates, instructors and others, e.g., newspaper and television interviews, formal laboratory write-ups and written summaries completed for class credit, collaborative writing of research paper or poster to present results formally. |
| Laboratory session (4 h) | Topic or action performed |
|---|---|
| 1 | Introduction–Faculty Lectures: 1) Presentation of scope of apprenticeship–biomedical engineering, BioMEMS and related applications; 2) Angiogenesis–tissue engineering, mouse aortic endothelial cells, topographical control and imaging; 3) Imaging techniques. |
| 2 | Discussion and presentation by laboratory manager on basic research skills, understanding risks and dangers, standard laboratory practices, and safety issues. |
| 3 | Introduction to cytometry techniques, including demonstrations and discussion of fluorescent labeling, apoptosis, MSDS, and literature searches. |
| 4 | Begin laboratory practicum. Cell culture techniques–nonadherent THP-1 cells. |
| 5 | Maintenance of cell cultures. Learn cell counting techniques including use of haemocytometer and coulter microparticle counter. |
| 6 | Cell culture techniques–adherent mouse endothelial cells. Prepare substrates and cultures for laser scanning cytometry experiment. |
| 7 | Laser scanning cytometry experiment and analysis. Prepare substrates for flow cytometry. |
| 8 | Flow cytometry experiment. Light microscopy and quantitative flow cytometric analysis of cytotoxicity. |
| 9 | Repeat flow cytometry experiment. Analyze results. |
| 10 | Review of results and preparation for presentation including media interviews. |
| 11 | Final discussion and poster completion. |
Study Participants
The students were preselected to participate in the program based on an application process involving interviews, letters of recommendation, or a recommendation from the director of the center. All students expressed an interest to learn state-of-the-art cell biology and biomedical engineering techniques. Most of the students had no experience in a graduate laboratory and no previous experience with cell culture.
The study included seven students, five males and two females. Of these students, four males and one female were in the 10th and 11th grade and one male and one female were in the 8th grade. All the students were classified as college bound. Four of the seven students were preselected by an application and interview process, and the remaining three received referrals from the director of the center. Students volunteered to participate in the course and received no grade or course-credit compensation. Of these participants, three were minority students.
During the program, students demonstrated the skills they learned, instructed the other student-learners, surveyed literature and material safety data sheet (MSDS) pertinent to the projects, and communicated their understanding of the project to the instructors during the laboratory exercises, media interviews, and while creating a final research poster presentation.
Study Evaluators and Course Instructors
Undergraduate and graduate students with demonstrated mentoring skills and an eagerness to participate in the study were selected to lead sections of the laboratory course through lecture and laboratory technique training. Lab managers and principal investigators advised the student instructors and assisted in the laboratory. Each session was led by a minimum of one graduate researcher and assisted by one senior researcher. The college researchers in biomedical engineering ultimately trained the participants to contribute to their research projects, using standard research tools and problem-solving skills to provide new data and contribute to actual research. The instructors participated in the final study evaluation process.
Study Data Collection and Analysis
Evaluations completed by the students and instructors at the end of the course are presented (Figures 5 and 6) and discussed. Students' impressions, comments, and challenges faced during the apprenticeship were also noted and discussed. Instructors' impressions were based on classroom observations, accuracy of data collected, skill evaluation, participation, poster presentation preparation, and informal and formal interviews.
Experiment 1: Flow Cytometry Study to Quantitate Cytotoxicity of Adhesive Test Materials
Rationale. Developing biomedical device technology often requires placing nonbiological components in a biological environment (von Recum, 1992). An important consideration to improve the biocompatibility and success of the implant is to preselect components that minimize or abrogate any toxic effects (von Recum, 1999). Cytotoxic effects are the preliminary test for biocompatibility, providing evidence of soluble contaminants or deleterious chemical interactions.
The primary goal of this project was to assess the relative cytotoxicity values of various adhesive test materials, cements and copolymerized glues. Quantitative flow cytometry was used to quantitate the ratio of viable cells after 24 and 42 h incubated in tissue culture wells coated with cured adhesive. Flow cytometry also identified the ratio of apoptotic cells to indicate the mechanism of cell death.
Materials and Methods
Test Materials. Test materials consisting of six chemical- and light-cured adhesives, cements, and elastomers. The bottom surface of an individual well from a 24-well culture titre plate was coated with test material, cured, and repeated in triplicate for each experiment. The test adhesives included a commercially available light-cured dental adhesive (C&B Metabond), a cyanoacrylate-based adhesive (Superglue), a chemically cured cement (Ross brand household cement), a light-cured methacrylate-based copolymer (BisGMA/TEGDMA), a chemically cured silicone elastomer (RTV Sealant), and rubber cement (Elmer's brand). A negative control consisted of cells incubated in polystyrene wells with no coating material; for an apoptosis-positive control, 12 μM camptothecin was added to additional uncoated wells 6 h prior to analysis.
Cell Cultures and Fluorescent Labeling. THP-1 cells (ATCC, Manassas, VA) were cultured, split, and then placed in each well in 1-ml RPMI medium at a seeding density of 0.5 × 106 cells/ml and incubated at 36°C under 5% CO2 and 100% humidity for each specified incubation period. THP-1 cells were used because they have a relatively short doubling period (a few days) and are a stable cell line for students learning cell culture. Cells were assayed for apoptosis in accordance with a commericially available apoptosis detection kit, propidium iodide (PI) and fluorescein isothiocyanate (FITC)–annexin V labeling Kit No. 3 (Molecular Probes, Inc., Eugene, OR). To explain the procedure briefly: cells were evacuated from the plates after incubation and placed in tubes on ice. Cells were washed twice with phosphate buffered saline (PBS) and centrifugation cycles of 5 min at 2000 rpm. Cells were resuspended in the Annexin V buffer available in the kit to provide 100 microlitre aliquots of 1× 106 cells/ml. Normal cell aliquots were titrated with FITC–annexin V to determine optimal staining ratios. During staining, cell aliquots were incubated with FITC–annexin V and propidium iodide stain for 15 min at room temperature, followed by quenching with 400-μl annexin V buffer solution. Cells were stored on ice until analysis within 2–4 hours.
Flow Cytometry. The flow cytometer (Becton Dickenson FACSCalibur, Franklin Lakes, NJ) was calibrated using the negative control cells, and regions of interest were selected or gated by virtue of their forward and 90° light-scattering properties. Flow cytograms were divided into quadrants using samples with single PI or FITC–annexin V labeling. Cells that stained negative for propidium iodide and FITC–annexin V were considered viable. Cells that stained positive for FITC–annexin V and negative for PI were considered to be in early-stage apoptosis.
Experiment 2: Laser Scanning Cytometery Study of Cell Growth on Micropatterned Silicon Substrates
Rationale. Adherent cell growth can be modified by the substrate surface chemistry and topography (Curtis and Wilkinson, 1997). These interactions are present in prosthetic devices, biomaterials, and other tissue engineering applications (von Recum and LaBerge, 1995; von Recum and von Kooten, 1995). By understanding the effects of these cell-surface interactions and by developing tailored substrates to control cell growth, many potential technologies can be realized. Fields that can be improved include cell culturing, biosensor technology, drug discovery and therapeutics, e.g., wound healing and cardiovascular therapies (Manconi et al., 2000), prosthetic devices, and micro- and nanodevice technologies that integrate biological and micro/nanoelectromechanical systems (bioMEMS and bioNEMS) (Mrksich, 1998; Ilic et al., 2000).
Various lithographic techniques including photolithography and chemical etching (Ilic and Craighead, 2000), microcontact printing (Ilic and Craighead, 2000) and soft lithography technology (Whitesides et al., 2001) can be used to generate micropatterned substrates. The influence of microtextured surfaces on cellular behavior is evident; however, very little is known about the fundamental mechanisms of this phenomenon (Curtis and Wilkinson, 1997). For example, in vitro experiments on micropatterned substrates have demonstrated that surfaces possessing microgrooves induce orientation of dermal fibroblasts, a phenomenon known as“ contact guidance.” In one study, the proliferation rate of the fibroblasts was changed by the wettability of the surface but not by the different topological micro dimensions on the surface (den Braber et al., 1996).
To analyze whether adherent cells react to surfaces with different geometric compositions, microgrooves were patterned onto silicon surfaces using standard photolithography and reactive-ion etch chemistry (Ilic and Craighead, 2000). Substrates with various dimensional configurations (groove width, ridge width, and groove depth) were created. The goal of this larger project was to quantitate and correlate the effect of micropatterned silicon substrates on the cell growth of mouse aortic endothelial cells (MAEC). As a preliminary project, the apprenticeship group examined the growth pattern of MAEC cultured onto photolithographic resist-patterned silicon substrates with a 10-micron square-grid waffle pattern (Fig. 4A) and compared the results to the microgroove observations (Moldovan et al.).
Materials and Methods
Patterned Substrate Surfaces. Silicon wafers were topographically patterned using standard photolithographic masking and silicon microprocessing techniques. Masks were created to produce a 10-μ scale waffle-iron pattern with square wells (Fig. 4A).
Endothelial Cell Cultures and LSC. MAEC from an established cell line (ATCC, Manassas, VA) were cultivated in T-75 (75 cm2) flasks in Dulbecco's Modified Eagle medium (DMEM) with 1% fetal bovine serum (FBS) and 0.5% penicillin-streptomycin antibiotic (PSA), split and incubated for 4–5 days to reach confluence. The surfaces of the patterned silicon substrates were sterilized with ethanol/water (95/5) and exposed to ultraviolet light for 30 min before seeding of cells. The monolayer of MAEC cultured in each T-75 flask was then dissociated using 0.25% x/v Trypsin-ethylenediamine tetraacetic acid (EDTA), centrifuged, and resuspended in fresh medium. Cells were seeded at a density of 30,000 cells/cm2 onto each substrate in a culture dish then incubated for 3–5 days at 36°C, 5% CO2, and 100% humidity. The cell substrates were placed on ice and fixed in ethanol then stained with PI for 30 min. The stained cell substrates were then imaged by laser scanning cytometry (Compucyte, Boston, MA).
Additional Experiments and Tools
In addition to these main projects, the students were introduced to other useful tools including a chemotaxis chamber, a Coulter microparticle counter, a haemocytometer, a fluorescence-activated cell sorter (FACS) sorter, fluorescence microscopy, and silicon and polymer microprocessing techniques and equipment.
RESULTS: EXPERIMENTAL DATA
Experimental Hypotheses
For the first project, students ranked the relative cytotoxicities of the adhesive test materials after surveying the literature, the materials safety data sheets, and product data sheets. Cytotoxicity levels were ranked again after culturing with the test adhesives and visual inspection of the cell cultures under a transmission light microscope. The images showed various cell morphologies that were used to approximate the effect of each test material. These qualitative rankings are presented in Table 3. The laser scanning cytometry results clearly illustrated to the participants the benefit of fluorescent labeling to more accurately quantitate and compare large populations of cells in culture. The students had mixed predictions for the patterning project. Most students hypothesized that the cells would reside within the microwells and within the microchannels.
| Students' rank based on MSDS | Students' rank based on microscopic examination of incubated THP-1 cells after 24 hours | Rank based on flow cytometric analysis of incubated THP-1 cells after 24 hours |
|---|---|---|
| 1. Silicone | Silicone | Silicone |
| 2. Superglue | C&B Metabond | C&B Metabond |
| 3. BisGMA/TEGDMA | Superglue | Superglue |
| 4. C&B Metabond | BisGMA/TEGDMA | BisGMA/TEGDMA |
| 5. Rubber cement | Rubber cement | Rubber cement |
Experimental Results
In this preliminary toxicity experiment, the flow cytometric cytograms (Figure 1) were used to quantitate the percentage of viable (Figure 2A) and early-stage apoptotic cells (Figure 2B). Figure 2A is a graph of relative “viable” cell percentages, obtained from cell counts that had both minimal PI and annexin V staining (lower-left quadrants of cytograms), following 24- and 42-h incubation with the adhesives. Figure 2B is a graph of the relative “early-apoptotic” cell percentages, obtained from cell counts that had minimal PI intake, but significant annexin V binding (lower-right quadrants of cytograms), following 24- and 42-h incubation. An experimental negative control, expected to have the maximum viable percentage, was obtained from cells cultured in the uncoated, polystyrene tissue culture plates. Variations between experiments were normalized by using these expected maximum levels (thick dashed lines), and relative toxicities were ranked according to these standards. The methacrylate-based BisGMA/TEGDMA and rubber cement had the lowest viabilities in both experiments, while silicone, Superglue, C&B Metabond, and household cement had lower cytotoxicities. Interestingly, the percentages of viable cells increased between the two experiments, which reflected an improvement in technique or protocol. The relative viability of household cement dropped slightly between the 24- and 42-h incubations. Surprisingly, that of the dental adhesive Metabond decreased to well below half the 24-h levels; however, the significant error leads one to question this result and repeat the experiment for better confidence.
Figure 1. Representative flow cytograms from two students' samples containing THP-1 cells labeled with both propidum iodide (PI) and FITC–annexin V intended to illustrate healthy versus nonhealthy cells. (A) This flow cytogram shows 11,068 gated events from cells labeled with PI and FITC–annexin V after incubation for 42 h in an uncoated polystyrene tissue culture well, a healthy, negative control. (B) This flow cytogram shows 11,529 gated events from cells labeled with PI and FITC–annexin V after incubation for 42 h in a tissue culture well coated with silicone. The observed low viability is not reflected in the average percentage viability (Figure 2A), which suggests improper cell handling during labeling or uncured material during incubation. The quadrant markings were established experimentally according to the limits of single-labeled samples of healthy THP-1 cells labeled with either PI or FITC–annexin V (data not shown). Cells in the lower-left quadrant of the plots have low PI and annexin V staining and are considered “viable.” Cells in the lower-right quadrant have low PI intake and high annexin V staining and are considered to be “early-apoptotic.” During the analysis, intact cells of interest are isolated (or gated) by virtue of their 0° and 90° light-scattering characteristics.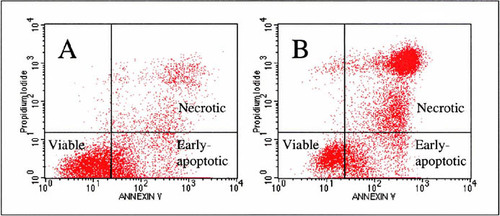
Figure 3 contains the individual flow cytometer adhesive results from one student. However, in this experiment, the number of viable cells was increased to include all cells with low PI intake, which includes both lower-left and lower-right quadrants of the flow cytograms.
In the silicon patterning experiment, endothelial cell growth was influenced by the micropatterned surface. The endothelial cells tended to localize to the inside of the individual squares of the waffle pattern, as shown in Figure 4B. These data support use of micropatterned silicon substrates to topographically control the growth of adherent cells. Micropatterned substrates provide an alternative means to culture cells, to interrogate individual cells or assemblies in culture, to study tissue–material interfaces, and to engineer cell growth in vitro (e.g., potential neovasculature analogs).
Experimental Discussion
Figure 1A and 1B plots were intended to illustrate cases of healthy versus nonhealthy cells. This particular silicone experiment (Figure 1B) had an unexpectedly high percentage of PI +/annexin V+ cells (late-apoptotic/necrotic), which is reflected in the large error bars in Figure 2. These preliminary flow data yielded inconsistent results and large standard deviations within experiments. These experiments were preliminary and the technique was new to some of theresearchers involved. Following the theme of the SGA, the researchers were working with the students on new projects, using new techniques, and sources of error (e.g., inadequate curing of adhesives) were identified during these early experiments. The students assisted in identifying potential sources of error, including suboptimal conditions during transport of the viable cells across campus to the flow cytometry facility for analysis, insufficient mixing of label and cells, and possible deleterious issues with uncured materials. (These issues, including steps to ensure adequate curing of the adhesives, were addressed in subsequent experiments with noticeable improvements [data not shown because completed after the SGA].) Table 3 compares the ranked, flow cytometry–derived viability percentages versus the microscope-based and MSDS-based student predictions. Interestingly, there is high agreement between the students' observed rankings and the flow data. The comparison of apoptotic induction was not dramatic, as shown in Figure 2B; however, Superglue had fewer early-apoptotic cells than those in samples with similar viability percentages. BisGMA/TEGDMA and rubber cement had significantly fewer viable and early-apoptotic cells, reflective of their high cytotoxicities. The other notable result is the low percentage of early-apoptotic cells in the camptothecin, “positive control” sample. It is apparent from the distribution of counts in the related cytogram that the camptothecin cells passed early apoptosis and the protocol should be optimized.
Figure 2. THP-1 cells were cultured for 24 h (black) and 42 h (gray) in the presence of various cured test materials. Cells were stained with PI and FITC–annexin V and analyzed by flow cytometry. (A) Cells that did not stain with PI, labeled “viable.” (B) Cells that did not stain with PI but did stain with FITC–annexin V, labeled“ early-apoptotic.” Cells incubated in uncoated polystyrene tissue culture wells served as the negative control. Early-apoptotic, positive control cells were incubated in uncoated wells and 12 μM camptothecin was added 6 h prior to labeling. Unfortunately, the positive control samples had low percentages of early-apoptotic cells, and their cytograms indicated that a majority of the cells had already progressed to late-stage apoptosis, thus the control protocol could be optimized.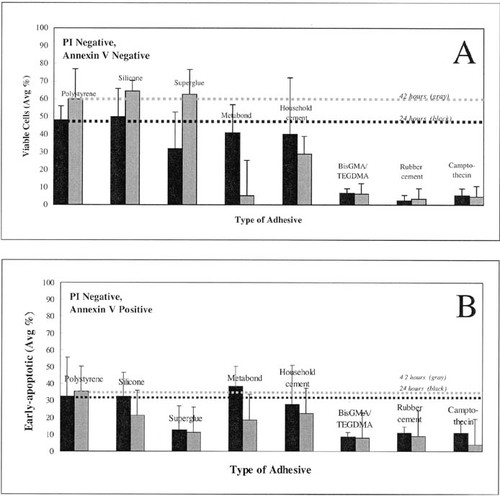
The waffle-pattern results were compared with those of a similar, more detailed LSC study in the laboratory concerning the effect of microchannels to create implantable “artificial capillaries” (Moldovan et al., 2001). In the related study, silicon wafers were micromachined to produce capillary-like microchannels (uniform and parallel grooves 10- to 150-μm wide separated by 100-μm ridges) in silicon and cultured with EC. Interestingly, the physical constraints were sufficient to induce the differential adhesion of EC in the grooves. A detailed LSC analysis revealed that the cells inside the grooves have different properties relative to EC on nonpatterned silicon (Moldovan et al., 2001). For example, an optimal groove width seemed to inhibit cell division and to favor differentiation. This study confirmed the feasibility of a microvascular tissue–engineering project and highlighted the unique capabilities of the LSC approach for combined microscopic and quantitative analysis of cells on large micropatterned surfaces. These findings support the benefit of extending the waffle-pattern study to obtain a more detailed and comparative LSC analysis of the cells grown on these substrates.
Figure 3. Graph and table of one student's flow experiment for THP-1 cells incubated with adhesives for 42 h. Viability, as measured here, includes both lower quadrants of the cytograms, where cells have only minimal PI intake and therefore overall larger percentages of “viable” cells than those shown in Figure 2A.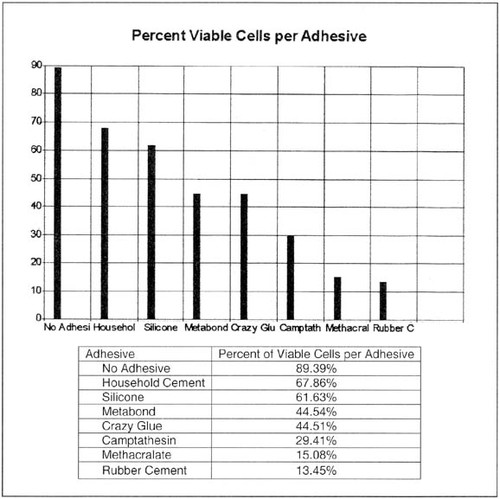
RESULTS: STUDENT AND INSTRUCTOR ASSESSMENTS
Instructors' Assessment of the Apprenticeship Experience
The following assessments of the SGA and participants' background, attitudes, and learning as it pertains to the course were derived from the Likert-scale evaluation presented in Figure 5, as well as observations of laboratory exercises, research activities, and formal and informal interviews. Alternative types of assessment of comprehension included informal and formal dialogues between students and researchers and media reporters. The students' understandings of the project were revealed in these interviews and recorded in various newspaper and television reports. These formal interviews assessed the students' abilities to synthesize the material and communicate it to both scientists and nonscientists. Students volunteered to participate and did not view the encounter as a formal test. The media coverage benefited the institution and the program by advertising the public service provided by the institution hosting the program as well as the research that was being explored.
Figure 4. LSC analysis of adherent mouse aortic endothelial (MAE) cells after culturing on a micropatterned substrate. (A) Micropatterned silicon substrate embossed with a 10-μm square-grid pattern created in photoresist on a standard 4-in. silicon wafer. (B) MAE cells stained with PI (nuclear stain) after incubation and fixation on the micropatterned substrate. Cells are shown growing in the wells of the micropatterned substrate.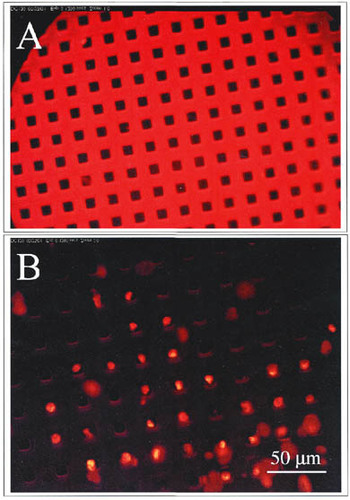
Instruction and Course Design. As evident in Figure 5, all mentors believed that the students were engaged during each of the experiments and left with a clear understanding of the experiment. The mentors also thought that the students benefited from learning and working with researchers from all levels. The mentors preferred to divide the teaching responsibilities into smaller modules as outlined in the syllabus. The mentors did not feel much frustration during their SGA experience, and they felt adequately prepared to teach their section of the course; however, a few would have preferred to have the students repeat some of the experiments (Figure 5). Most of the mentors thought that seven students was a manageable instruction group, and only one mentor thought that the group of seven was too large for the laboratories.“ A smaller group would be more personal and provide each student a chance to be more involved, but seven wasn't unmanageable,” stated one mentor on his evaluation. In general, it was observed that the average group of seven students was usually managed by two mentors. One instructor demonstrated the procedure, and at least one other was preparing materials, setting up equipment, assisting other students, and being available in case of an emergency. Three of the five mentors preferred to break the students into smaller groups of two to three students, and only one mentor disagreed. All the mentors highly enjoyed working and learning with students between grades 8 and 11, and most of the instructors did not think that mixing 8th and 11th graders was problematic to the success of the course. Four of the five mentors preferred that the students work in small groups instead of working independently (Figure 5). The one undecided mentor added that although “group input is a benefit to everyone, I think students would participate more actively as individuals.” Overall, the mentors believed that the group of seven students was acceptable for the SGA laboratories (Figure 5); however, one mentor noted that “smaller groups of students, for example, two to three students, are better for safety during laboratory procedures where dangerous materials, for example, propidium iodide, must be controlled or where space is limited as in the flow cytometer room.”
Various experimental procedures were accomplished with two apprentices and one mentor. Although no formal assessment was made to compare various subgroup sizes, a 2-to-1 apprentice-to-mentor ratio seemed to occur naturally and most frequently during performance of procedures. This student ratio was most likely preferred given the limited working space available around most equipment, for example, in the culture flow hoods, the flow cytometer with only two possible activities, sample loading and computer collection, and with regard to providing optimal hands-on experience, as well as optimal personal instruction, and allowing students to teach each other. Peer instruction was often observed during these two-student scenarios when one student skilled in a procedure instructed the second student on the proper technique while the instructor monitored the interaction and corrected the student when needed. Successful peer instruction was observed during the cell culture procedures, the cell counting procedure, and the flow cytometry analysis. In these instances, students successfully instructed each other, and the mentor-observer monitored the students' technique and comprehension of the important steps of each procedure. From these interactions, mentors also observed that with proper initial training, the students continually trained other students while practicing proper technique throughout the course (e.g., cell splitting and cell counting). It was observed that student learning appeared to be secondary to increased student access, especially for the quiet and the younger students. The older or gregarious students tended to dominate the SGA activities and discussions (e.g., the two students spotlighted in the television news report were the older, outgoing students who similarly dominated laboratory activities; WCMH-TV 4–5 p.m. NEWS, 2001).
Although not measured in the assessment, communication among the mentors and the students was integral to the success of the SGA. The mentors and students constantly received feedback throughout the SGA. The mentors notably observed that the students spoke freely to one another and to the mentors throughout the course. Collaboration among the participants was also noted during the SGA. For the flow cytometry project and final poster presentation, out of necessity, smaller groups of two to three students completed sections of these projects. For the flow cytometry experiment, each student`s results were compared with those of the group and statistically averaged to indicate any variations in technique. It was difficult to interpret the flow cytometer toxicity data effectively on the computer given the large volume of data and large group of students; however, by laying each data plot out on a table, qualitative trends were easier to identify and it was easier to move data around for comparison. The mentors observed that small groups of participants sorted through the plots and were able to make associations between plots that were not apparent to individuals at the computer.
Figure 5. Results of mentors' Likert-scale assessment of SGA experience and background.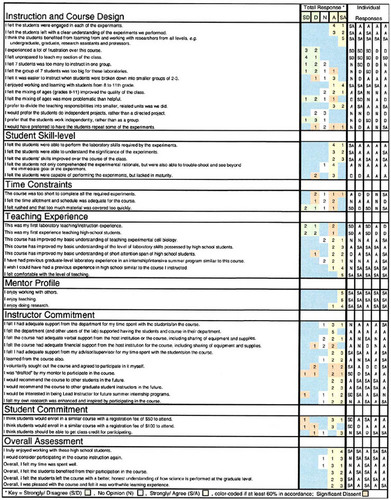
Student Skill Level. At each laboratory session, students successfully exhibited cell culture techniques, including accurate pipetting, sterile technique, and cell splitting. “We cultured cells in a petri dish and learned how to grow them and keep them alive,” recapitulated one student to the interviewer (Dohrmann, 2001). All instructors agreed that the students adequately performed the laboratory skills required by the experiments (Figure 5). They also agreed that the students were able to understand the significance of each experiment (Figure 5). The mentors agreed strongly that the students' skills improved during the course of the class (Figure 5). By following the SGA schedule, participants practiced their cell culture skills at each meeting, and by comparing cells under the light microscope at each session, they successfully gauged their progress and improved their technique throughout the course, according to the mentor-observers. Four of the five mentors thought that the students were able not only to comprehend the experimental rationale, but also to troubleshoot and see beyond the immediate experimental goal (Figure 5). The dissenting observer thought that the “students don't understand the ramifications of not being accurate or precise.... They don't think to the end of the project always” (mentor evaluation). “I feel that this was a very intelligent group of students... compared to me in high school,” remarked another mentor. Three of the five mentors agreed that the students were capable of performing the experiments but were lacking in maturity (Figure 5). “It seemed like the younger students were actually more attentive than the older ones” (mentor evaluation).
Introduction: Adhesives are a very important part of many new procedures and novel devices used in and on people to aid their health. They keep small implants together and have been used as more practical“ stitches.”... Unfortunately, many adhesives are potentially cytotoxic, leeching chemicals that kill cells and harm the body. As adhesives are used increasingly in the body, it has become important to evaluate their ability to kill cells. Using new flow cytometry technology, we now have an efficient and accurate technique to measure the cytotoxicity of adhesives by measuring the amount of apoptotic cells using annexin V and propidium iodide, two chemicals that mark apoptotic cells, when the cultures are incubated with an adhesive material.
As mentioned, part of the research-skill assessment included the group's completion of a formal presentation board at the end of the course. The students worked independently and collaboratively to analyze data and write sections for the poster. After completing the poster presentation, the students demonstrated a clear understanding of the purpose and details of the two main projects. The following is an excerpt from one student's contribution to the introduction:
Results: The percent of cells which died in each experiment varied greatly between the seven adhesives tested. The control experiment, which did not contain any adhesives, had a 10.61% mortality rate. 89.39% of these cells tested either positive for Annexin V and negative for Propidium Iodide or negative for both of these factors. The cells in the silicon experiment exhibited a 38.37% mortality rate and 61.63% of these cells tested negative for Propidium Iodide. Household Cement had similar results with 67.86% of the cells testing negative for Propidium Iodide. Two of the Metabond experiments showed very different results. One of these experiments showed 60.7% of the cells testing positive for Propidium Iodide while the other experiment had only 20.8% of the cells test positive for the same agent. Of the cells in the Crazy Glue experiment only 44.51% of the cells tested negative for Propidium Iodide, which means that a majority of the cells tested positive for PI. Both the rubber cement and methacralate [sic] had a very high percentage of their cells test positive for both Annexin V and PI with 84.97% and 83.11% testing positive respectively. The camptathesin [sic] experiment showed a mere 9.65% of the cells testing negative for both PI and Annexin V. The experiments which put cells without PI and Annexin V through the flow cytometer all showed similar results. These experiments all showed [data counts in a plot along] a 45 degree line from the bottom left corner of the graph and tapering off just shy of the center.
Discussion: The data suggests [sic] that the order of the biocompatibility for the adhesives is as follows (from most to least comparable): household cement, silicone, Metabond, Crazy Glue, camptathesin [sic], methacralate [sic], and rubber cement. This list was derived by comparing the percent of cells which tested negative for PI for each adhesive. Propidium Iodide binds with the DNA of necrotic cells so by distinguishing the presence of cells which tested positive for PI the toxicity of the adhesive can be determined. Another chemical used in this experiment was Annexin V. It binds with the phosphatidylserine (PS) found on the outer membrane of cells that may be headed towards apoptosis. The PS is thought to signal macrophages and induce phagocytosis of apoptotic cells. Both of these chemicals fluoresce in the presents [sic] of the 433 nm laser of the flow cytometer. By using PI and Annexin V one is able to assess how certain adhesives affect cellular necrosis.
In order to insure the quality of the cells tested in the flow cytometer it is necessary to test only a certain part of the sample. This is done by“ gating” a group of cells by virtue of their forward and 90 degree light scatter characteristics. The granularity and the size of the cells tested is important in guaranteeing the quality of the experiment`s results. Another factor important in insuring good results is eliminating error caused by any other agents in the sample which may fluoresce in a similar way as the Annexin V and PI. This is done by putting the sample without PI or Annexin V through the flow cytometer and then erasing the results of this test from the results of the test which had Annexin V and PI. These tests always result in a 45 degree angled line which ends just short of the center of the graph.
One interesting thing which can be observed from some of the results is the presence of two horizontal parallel lines in the top third of the graph. The top line is shorter than the bottom one and has double the concentration of PI. This larger concentration suggests that it died with a higher amount of DNA than those on the lower line. Thus this is interpreted to mean that the cells in the two lines died in different cellular stages. The ones with the higher concentration probably had double the amount of DNA because they were about to divide. One interesting follow up experiment would be to observe these cells under a LSC so that they could be observed visually and their differing cellular stages could be confirmed.
The students worked with the senior researchers to analyze and interpret their data, to review relevant literature, and to revise their poster presentation. The following is another student`s results and discussion section from the same experiment with accompanying graph in Figure 3: During this formal writing exercise, the students adequately conveyed their findings; however, their writing was possibly hindered by a limited “scientific vocabulary,” as observed by the mentor who assisted during the assembly of the poster. Thus, during the analysis and write-up, the mentor provided the students with scientific terms (e.g., significant, quantitative, qualitative, comparative, augment, diminish, suggest, etc.) to improve the quality and scientific merit of the poster.
Time Constraints. The mentors were divided on the issue of adequate length of time for the course (Figure 5). Three of the five mentors thought an adequate amount of material was covered during the course at an adequate pace, and one mentor felt rushed (Figure 5). One evaluator asked that students be allowed to repeat experiments. When fewer experimental procedures were required during a session (e.g., during the LSC project when students worked together and performed fewer steps), the students asked more questions, possibly having had more time to digest and analyze the results. The pace during the flow cytometer experiments was busier, with many short, repeated procedures. During these experiments, the students were quieter and more engaged during each procedure, and fewer questions were asked.
Having run out of time to complete the analysis and poster, five students returned to the facility after the formal apprenticeship program ended to work independently with a mentor to complete the project and analysis. During these one or two student meetings, each student discussed the significance of the procedures in greater detail than during the SGA. The individuals contributed to the analysis, summarizing results and identifying potential sources of error. The mentors discussed data presentation in terms of selecting appropriate tables and plots, and the significance of accuracy, error limits, and other considerations to illustrate findings more effectively. Although these individual meetings were not scheduled to occur during the SGA, the extra time and meetings were needed for proper data analysis and assembly of the formal poster for the upcoming National Symposium on BioMEMS and Biomedical Nanotechnology held September 22, 2001, in Columbus, Ohio.
During a joint, post-SGA afternoon meeting to complete the final project, the students requested one mentor to advise their poster-writing activities. The mentor observed that inadequate time was allotted during the course for student discussion and analysis of results. According to this observer, although the students were successfully able to perform the activities during the laboratories, some essential points pertaining to the study and procedures were missed and realized when the students started compiling the poster and comparing their results. “Extra time spent analyzing the results during the course would have decreased the time and effort spent completing the final poster,” commented one mentor. Given the time constraints placed on these post-SGA sessions, the imminent poster deadline, and their inexperience, the students orally expressed significant unease during this process, asking the mentor for guidance on how to outline the poster contents, how to display the data for interpretation, and how to identify the important results. During the process of making the poster in a group-mentor setting, the students learned how to graph and display data effectively, how to organize the results, and how to work collaboratively to divide sections and assemble the poster in a short time. The success of this activity was evidenced in a well-organized and instructive poster. Some of the data presented in the poster were useful to a conference attendee, and the authors were asked for a formal publication citing the results for further reference. Moreover, many conference attendees commented on the scientific merits of the poster, inquired how to get their children into the program, or asked for advice on replicating the program at their institution (refer to quote in Conclusion).
Teaching Experience. All five mentors were comfortable with the level of instruction during the course (Figure 5). Three of the five mentors had experience instructing laboratories, and four of the five had instructed high school students previously (Figure 5). The other mentors lacked this experience. All mentors agreed that the SGA course improved their basic understanding of teaching experimental cell biology and of the level of laboratory skills possessed by high school students. Most of the mentors acknowledged the short attention span of the high school students relative to that of most graduate students. Three of the five mentors had former experiences in a laboratory setting similar to the SGA presented, and all five would have preferred to have had experienced this SGA during high school (Figure 5).
Mentor Profile and Commitment. All five mentors stated that they enjoyed working with others, they enjoyed teaching, and they enjoyed doing research (Figure 5).“ I was happy to have students ask about real world applications of the experiments” (mentor evaluation). Three of the mentors volunteered to participate in the course, and three were asked by their advisors to participate. All five mentors would recommend the course to future students and instructors. Only one mentor was not interested in becoming the Lead Instructor. Most of the mentors believed that they had received adequate support from their department and advisor/supervisor for their participation in the course. They all agreed that the department and other users of the laboratories supported having the students and the course in their department. Most of the mentors thought the course had adequate verbal support from the host institution for use of equipment and supplies; however, adequate institutional financial support was not agreed upon. Interestingly, most of the mentors were also educated during the course, and they thought that their own research was enhanced and inspired by participating in the program (Figure 5).
Student Commitment. One mentor stated in his evaluation,“ The students were very eager to learn.” According to Figure 5, most of the instructors were willing to charge a $50 or $100 registration fee for materials and other course costs in future SGAs; however, a few mentors strongly disagreed. Three of the five mentors thought the students deserved class credit for their participation, but one mentor disagreed (Figure 5). “The students were eager to participate in the program,” observed one interviewer in a related newspaper report (Dorhmann, 2001). The students seemed committed to the course, attending each session regularly, with absences due only to work- or family-related commitments. Students were prepared for each session, bringing extra materials, including lab coats or household materials, when needed. Students readily volunteered to perform procedures, and instructors observed that they were eager to complete the projects properly and willing to repeat the experiments and reanalyze their results. The students stated throughout the course that they were excited by the potential of having their results published in the form of a scientific poster or paper. Through these statements and their excitement about publishing, these middle and high school students apparently recognized some benefit of gaining publications toward their academic future. Many of the students were college bound and noted their participation in the course as an achievement for their college applications. Furthermore, six of the seven students had at least one parent who was a scientist and likely shared their interest in starting a publication record.
Figure 6. Results of apprentice/participants' Likert-scale assessment of SGA experience and background.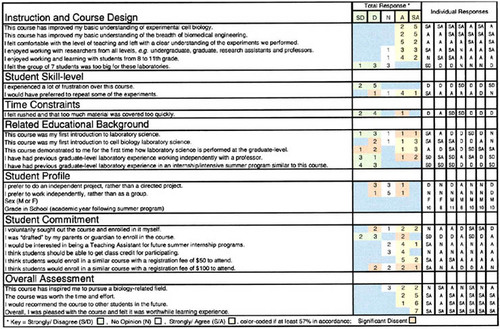
Overall Assessment. Overall, all five mentors were pleased with the SGA course and thought that it was a worthwhile learning experience. In addition, all five mentors truly enjoyed working with the high school students (Figure 5). Four of the five instructors agreed to participate in the course instruction again and believed that their time was well spent. Finally, all five mentors thought that the students benefited from their participation in the course and that the students left the course with a better, honest understanding of how science is performed at the graduate level (Figure 5).
Students`Assessment of the Apprenticeship Experience
Many of the following assessments were derived from the Likert-scale assessment in Figure 6 and written evaluations, organized to compare the participants' background, attitudes, and learning as it pertains to the course.
Assessment of the Instruction and Course Design. All participants stated that the SGA improved their basic understanding of experimental cell biology and their basic understanding of the breadth of biomedical engineering (Figure 6). All participants felt comfortable with the level of instruction and left with a clear understanding of the experiments performed (Figure 6). None of the students had ever participated in an internship or intensive summer program similar to the SGA described here (Figure 6). In accordance with the beliefs of the mentors, none of the students thought that the group of seven students was too large for the laboratories (Figures 5 and 6). The joining of middle and high school students, as well as mentoring from researchers of all levels within the university setting, was enjoyable to all the participants (Figure 6). One student summarized his SGA experience and observations, confirming some of the SGA aims:
This summer, I had the opportunity to work with a group of intelligent and interesting students, graduate students and researchers during my internship at [the university].... All of these people have very interesting jobs and have given me a glimpse into the field of work I hope to pursue.... [One laboratory manager] is in charge of a very expensive machine called the flow cytometer, which can count and sort cells at a tremendous rate, about 10,000 cells per second, by using lasers and chemical markers. This machine plays a crucial part in some of the experiments we do. [The manager] taught us how to use the $250,000 instrument and allowed us to operate it for our adhesive experiment. [Some graduate students] perform experiments in nanotechnology and biotechnology, gaining expertise in their fields that they will use when they become researchers and scientists. Finally, [another laboratory manager] runs the lab, helping students learn and deal with the problems they face in their studies.
With respect to restructuring the course, one student recommended“ maybe doing more experiments, even if they are a little simpler for first timers and get progressively harder for veterans.” Because the SGA program occurred during summer vacation, many students were absent for as long as 1 week for other commitments and family obligations. In the current SGA schedule there was no time allowed for repeating or making up experiments. One participant suggested that future sessions “meet more, over a more condensed period.”
All students “felt comfortable with the level of teaching (mentoring) and left with a clear understanding of the experiments performed” (Figure 6). “We got to learn a lot about science and we got to see and use a lot of cutting-edge technology,” remarked one student during an interview (Dohrmann, 2001). The following summary of the SGA experience was submitted by one of the older participants for review before it was submitted to the student`s host high school as part of a separate career outreach requirement:
From the students [sic] perspective this [flow cytometer] experiment and the entire program provided invaluable laboratory experience. The experiment deepened the participants' depth of knowledge by exposing them to complicated procedures, complex machinery, new laboratory techniques and a helpful university staff. The opportunity to work with such equipment as the LSC and flow cytometer gave students a better understanding of what tools are used at university level research. This equipment also taught the participants more about how cells grow and eventually die. Besides the special equipment many other factors contributed to the success of this program. The most important of these being the incredible staff of researchers and graduate students who aided the high school students throughout the course. Before the program many of the students had believed that most of science had already been discovered, and subsequently had never performed an experiment where the result was not already known. The staff at [the university] changed these students' perception of science dramatically. They taught the students about the experiments they were working on and the problems that they were trying to solve. The researchers introduced the participants to new experiments and helped them follow a seemingly cryptic series of instructions. This way of learning science is much different from how the subject is taught in school. In many ways this method is more affective [sic], because this method gives students a deeper understanding of the science by exposing them to practical applications of the subject. Although it would not be pragmatic to teach this way to every student, for a select few this type of experience works with tremendous success.
The apprenticeship exposed the students to the realities of working in a true research environment (e.g., certain cell cultures died unexpectedly, and, in one instance, materials set aside for one experiment were accidentally used by another laboratory, which delayed completion of the experiment). The opportunity to work with such advanced equipment as flow and laser scanning cytometers provided the students with a more realistic impression of biomedical research. As one student remarked to a reporter, “It's definitely not the stuff we get to do in high school” (Dohrmann, 2001). Using high-tech tools, the participants learned about basic cell biology, cell death, and quantitative cell biology techniques. One mentor noted that the students were exceptionally intrigued by the silicon and polymer microprocessing techniques used to generate the LSC substrates, hence their interest in microfabrication applications in cell biology, or tissue engineering. In addition to accessing high-tech equipment, the participants expressed great enthusiasm while working with the younger mentors, engaging freely in joking and asking scientific and nonscientific questions.
Student Skill Level. None of the students felt a lot of frustration during the course (Figure 6), which suggests that they thought that the required skills were within their abilities and/or that they were given ample time and guidance to learn the appropriate skills. Five of the seven participants would have preferred to repeat some of the experiments (Figure 6); however, it is not clear whether the repetition was wanted as a way to improve skills or simply to perform more research. This apprenticeship course was designed to be a practicum— therefore, no objective tests were given. A majority of the students stated at the beginning of the course that they preferred written reports in lieu of formal tests, and because formal grades were not required, the Likert-scale assessments, personal observations and interviews, and final presentation board were acceptable.
Time Constraints. Only one participant thought that the course was rushed and covered too much material (Figure 6). As evident in the written statements that follow, the students unanimously requested more time in the future to repeat and continue the experiments. When asked how to improve future SGAs, the participants responded as follows: “Longer time! It was fun!” “Repeat the experiments, definitely.... Make sure that everything's organized ahead of time so as to not waste any time while you're there.” “I think you should maybe know what's going on for the next week at least the week before and maybe have more time.”“ Maybe more meetings/time, more of an idea of what you will be doing when you sign up.” “More time to learn, so more experiments can be done and more materials can be handled.” Such a continuation of experiments would require more time and supplies; however, it would provide more data and aid the participants' and mentors' assessment of performance and skill improvement.
Related Educational Background. As evidenced from Figure 6, four of the seven students had former laboratory science experience and only two had formerly been introduced to cell biology laboratory science. Four of the seven participants stated that this course demonstrated to them for the first time how laboratory science is performed at the graduate level (Figure 6). Three of the seven students stated that they had previous graduate-level research experience working independently with a professor; however, none had participated in an SGA program as described here (Figure 6). In addition to the data presented in Figure 6, the following are student descriptions of previous science coursework and laboratory experience that they thought would be pertinent to this biotechnology SGA. It appears from these excerpts that many of the students have limited practical laboratory experience performing cell culture or using high-technology laboratory equipment. Many of their former research studies were independent science fair projects. Most of the students focused on formal classroom/laboratory courses at the high school and some at the college level.
Apprentice 1: “In school we have covered much of the basic biology areas, including cells, DNA, RNA, immune systems, Mendelian genetics, evolution, mitosis, meiosis, and molecular level studies in a few areas.... Also in terms of chemistry we have done reactions, acids and bases, physical chemistry, atomic level chemistry and stoichiometry. I have a good background for DNA, genetics and cell biology as we covered those rather well. I should be able to pick up concepts quickly because we were exposed to a multitude of topics on the basic level.”
Apprentice 2: “As a seventh grader, I took life science which involved learning basic cell structures (of plants and animals/humans) and what they were composed of. Unfortunately, I haven't had biology yet. I`ve also had an introductory course on chemistry, which included acids, bases, titrations, elements, so on and so forth. Also, I have been involved in science fairs since I was in fifth grade. Mainly, I have worked with total plate counts of bacteria. For the past three years I have been working with total plate counts of bacteria found in commercially prepared meat samples. I have mainly been looking for overall trends, but I did work in a hospital lab to identify specific bacteria for some of my samples. Science fair has also given me the knowledge of the scientific method.”
Apprentice 3: “In high school I have taken both biology and chemistry courses. The biology course covered both molecular biology and the study of plants and animals. We performed several laboratory experiments. We made cell cultures many times and worked with many types of cells. I took the AP test which covered both molecular biology and plants and animals. As a sophomore I took an honors chemistry course which covered inorganic chemistry. I was in the lab 2 out of every 6 days of the class and gained a lot of laboratory experience....”
Student Profile and Commitment. The student participants had no collective preference for working independently or under a mentor's direction, or working independently versus as a group (Figure 6). The participants' ages and genders are listed in Figure 6 along with their individual responses for further analysis. Many of the students thought that they should get high school credit for participating (Figure 6), which, they added, would also allow them to invest more time in the apprenticeship. In an amended evaluation of one participant, “The course should equal some type of high school science credit.” Many of the students were interested in serving as mentors or teaching assistants for future SGA programs. Although many of the students were referred by their parents, who worked at the university, only two felt compelled by their parents to participate in the SGA (Figure 6). Although this SGA was provided at no cost, students agreed that $50 was a reasonable enrollment fee, but $100 for the 11-week (44-h) experience seemed excessive to many of the participants.
Overall Assessment. From the data in this assessment, the students unanimously and strongly agreed that the SGA, as presented here, was a worthwhile learning experience, worth their time and effort (Figure 6). Although only four of the seven students were inspired to pursue a biology-related field following the course, all the students would recommend a similar SGA to their peers (Figure 6). The written evaluations of various SGA participants yielded the following comments:“ Overall the class was a great experience for me and taught me many new things about science.” “I really enjoyed everything. It was worth all the time I spent there or getting there.... Can I do it again?”“ All in all, I really enjoyed being in this program and hope that it can be continued in the future. I gained valuable insight into what it takes to make it in the field of science and what fun it can be working with my friends in science. Next to this, high school science will be a piece of cake.”
DISCUSSION: MAJOR FINDINGS AND FUTURE IMPLICATIONS
There are many principles historically associated with apprenticeships. These principles include formation of close relationships with mentors, learning thorugh doing authentic exercises, using authentic tools and techniques, and learning within a community that values similar practices. The SGA described in this study was designed to provide these principles.
The students and instructors quickly formed close relationships during the course and as a result, they were highly engaged throughout the SGA. The students were excited to participate in actual research. They were very excited about the challenge of having their data potentially appear in a research publication. That the results would be part of a larger research project further piqued the students' interest, concentration, and commitment level. The instructors were equally engaged, energetic, and interacted very well with the students. The students spoke freely with the instructors, asking many questions.
The researchers or master craftsmen were devoted mentors to the students. Working alongside the researchers, the students were excited to learn the results of an actual problem. The researchers and students addressed each research challenge together. They taught the students about the experiments they were working on and the problems that they were trying to solve. Quoting one student, “The researchers introduced the participants to new experiments and helped them follow a seemingly cryptic series of instructions.” They also taught the students how to apply the scientific method to a variety of problems. This SGA-style of learning science is much different from how the subject is taught in high school. The instructors focused on following a good scientific method, revealed “failed” experiments and unclear results, potentially changing these students' understanding of how scientific research progresses outside the classroom. In many ways, this method is more realistic because it gives students a deeper understanding of the science by exposing them to practical applications where they are forced to work in a team environment.
Biotechnology: A Curioso-Attractant for Students
Biotechnology has been identified by other educators to attract students' active involvement and interest when applied to classroom learning (Brown, 1999). Students found the subject matter easier to learn when it related to their lives. Furthermore, evidence indicates that a science curriculum, which helps pupils to see how science relates to their lives, has a positive impact on their attitude to science (Bennett, 2001). The researcher in one study developed a unit on biotechnology including recombinant deoxyribonucleic acid (DNA), plasmids, and methods for DNA fingerprinting to supplement a section on DNA and protein synthesis in a high school biology course. “The concepts associated with biotechnology tend to be mysterious... bordering science fiction,” and therefore interest many students. Without the necessary equipment, the instructor is limited to describing the concepts and invoking current events or biotechnology-related social issues. In this context, the only limitation is time and energy.
Biomedical engineering excites students interested in medicine and developing new materials and therapeutic devices. There is no conventional course in this applied subject at public high schools, thus students are eager to investigate this new field where they can apply laboratory skills from chemistry, physics and biology. They are delighted to realize they can contribute to research directly. They also appreciate the complexity of the research and by using the scientific method advances can be made. A similar public interest in this research is reflected by the television and news reports that were interested in the SGA and the studies they were doing.
Teaching Reform: Apprenticeship Programs and Related Issues
Historically, apprenticeships have been the modus operandi to train pupils to master a special craft or skill. It is expected that learning these skills and techniques through practice and instruction will require a relatively long period of time as was required to train the skilled master. Intensive submersion can expedite the transfer of knowledge; however, skillful expertise generally requires a longer time commitment. In addition to having a skilled instructor, the pupil is required to step into the master's workshop and use the proper tools. It is in this setting that the pupil learns the trade.
The educational strategy in support of apprenticeship learning is comprised of science educators who advocate for active students performing scientific investigations. The investigation focuses on actively engaging students in authentic scientific inquiry; however, the students can be in an artificial setting. An apprenticeship provides the actual setting and situates the investigation in the context of the specific research community.
Barab and Hay (2001) characterized the experiences of 24 middle school students who completed a similar 2-week long “Science Apprenticeship Camp” (SAC) designed for students to work with real scientists engaged in real research. The SAC study was unlike other studies about laboratory apprenticeships that focused either on making the classroom more apprenticeship-like or on apprentices working for extended time periods directly within a community. In the SAC, students worked directly in a laboratory in groups of four for a relatively short period. The students who participated in the SAC were noted as being inspired to study science and engineering as a result of their experience.
A certain amount of autonomy is imparted to the students when they are contributing to an active science project, and they perceive themselves as contributing to real scientific work. This autonomy is reflected in a sense of commitment and ownership of the project. The small-group apprenticeships create a working partnership between scientists, science educators, graduate and undergraduate students, and the participants, working on a project in which they have an invested interest (Krockover et al., 2001).
Individuals learning in the context of the classroom are not exposed to the mature field of science—a practice that uses the information being learned (Barab and Hay, 2001). In a research setting, students identify the cultural identity and community associated with being a scientist and authentic situations in which a skilled researcher is useful, e.g., studying diseases and engineering therapeutic drugs.
Team instructing/mentoring provides a way to manage multiple students' goals, interests, and projects simultaneously. Furthermore, coteaching is a highly constructive, mentoring environment for prospective research teachers (Tobin, 1999).
Trends in Science Education Reform
An apprenticeship-style course models experiences in research laboratories and better reflects trends in educational reform (Henderson and Buising, 2001). Traditional high school courses expect students to transfer what they learned to novel and real-world situations. Students are more likely to remember and draw upon that which is familiar. Most traditional laboratory classrooms expect students will transfer classroom activites and concepts to extracurricular activities. However, advocates of educational reform often describe classrooms as inauthentic in which most activities are structured around artificial contexts for learning.
A related study was conducted at a large Midwest university to understand how students perceived their learning environment (Nicaise et al., 2000). In “inauthentic” classrooms, students' energy during tasks diminished and students remembered concepts at a superficial level. When authentic contexts for learning were applied, many successful students in the study described their classroom as “fun and exciting with real-world relevance.” It should be noted that students who did not share this viewpoint were not as successful in the course. Practical laboratory exercises enable students to gather data using all of their senses. In a laboratory setting, students are able to use their powers of observation and inference. These skills of observation and reasoning are part of a scientist's personal toolbox. Practical exercises thus play a pivotal role in developing a scientific mind (Delargey, 2001).
Research-based, student-centered, and cross-disciplinary course structure reflect the typical situation encountered by scientists. In apprenticeship partnerships, participants are required to perform similar team projects through collaboration with corporate or academic research institutions. Participants mimic scientists by identifying, developing, and possibly funding a project, then conduct the experiments and eport the results. Through these apprenticeships, participants will practice the scientific method and learn some of the critical facilities expected of an educated scientist.
CONCLUSION: WHAT ARE THE LIMITATIONS AND SHOULD THIS METHOD OF APPRENTICESHIP INSTRUCTION CONTINUE?
Experimentally, the students produced useful research information and learned modern techniques and skills in cell biology. By the end of the program, participants without previous cell culture experience successfully and reproducibly cultured nonadherent and adherent cell lines, completed measurements with assistance using flow cytometry and laser scanning cytometry, and performed the preliminary data analysis at the level of college researchers. The students' cell culture skills improved throughout the apprenticeship. The cytometry results demonstrated the students' satisfactory laboratory skill level associated with cell culture. Each student expressed an interest to repeat more experiments and the students seemed to learn by trial and error.
Pedagogically, the apprenticeship program generated a productive synergy between university- and secondary-level students, advancing the students' laboratory skill base and exposing them to a research environment. The research setting constituted a rich environment for participatory science learning. Most secondary schools are not equipped physically or financially to provide similar research experiences; therefore, organized apprenticeships in cooperative research institutes, are an excellent alternative. Small-group apprenticeships provide a means to accommodate more students using a limited expenditure of time and supplies. A small group of interested and selected participants can benefit from this type of laboratory experience, and the host institution is able to provide community outreach and mentoring in a compact, organized format.
The following are the comments from a researcher at the International Symposium on Biomedical Nanotechnology where a poster describing the program and results was displayed:
For me [the important issue] is of how we should change curriculum and course works and lab works at the college level so that students who have interest and have great talents could become great scholars, scientists and engineers... I have been mentoring many graduate and college students in my lab for the last ten years or more and I see a lot of deficiencies that we could remedy... to fit into this emerging but too fast accelerating technology and science. For this reason, I am quite interested in your program. As I get older and older, my mind is getting more and more towards how to uplift and [improve] education for the younger generation.
However, by identifying research opportunities that only slightly extend their current research commitment, researchers can provide a rare real-time research learning experience. Clearly, the minimum requirements in establishing such an apprenticeship program are obtaining institutional access, securing adequate laboratory space and selecting a coordinator for the program. The coordinator then defines the program aims, identifies specific projects to be addressed, and selects the participants and researchers. Once organized, the apprenticeship is relatively self-sufficient. Organized properly, both the research institution and the participants can achieve positive results.
The most important requirement, and likely the greatest limitation, is the personal time commitment of the researchers. High-tech is fun and exciting; however, it costs. Researchers who appreciate alternative learning environments will be more likely to financially support interinstitutional research opportunities similar to the one presented in this study. Additional support systems may be required for these“ authentic” learning environments to succeed and persist. Further studies need to be completed to establish whether apprenticeship learning is cost effective, and if not, mechanisms to make it affordable. Financial costs associated with hosting a small group of students can be minimized by having the students work on active projects, using supplies and costs already designated to that project. In addition, public and private research institutions are now providing competitive seed money to promote similar youth education and outreach programs.
FOOTNOTES
Monitoring Editor: Erin Dolan
ACKNOWLEDGMENTS
We want to thank David Feeley, Giacomo Ferrari, Angelica Liu, Jacob Liu, Arnab Majumden, Jordan Ruben, Amy Schlegel, David Briley, and Nicanor Moldovan for participating and contributing to this study.



