Video Views and Reviews
Some impressive video movies have appeared as supplemental material in recent issues of the Journal of Cell Biology (JCB), and in this article I review several of them. In general, the JCB format provides each video clip with its own caption, in addition to any contextual references in the article itself, and a separate descriptive section—designated Online Supplemental Material (OSM)—at the end of the article summarizes the caption detail. Thus, although the“ supplemental” videos are usually well-integrated research records, many can be appreciated as stand-alone records, often with minimal reference to the parent article. The JCB videos are also available through a separate “Supplemental Material” list, which, unlike the articles, may be viewed by nonsubscribers. The list may be accessed at http://www.jcb.org/supplemental. Unfortunately, this entry route provides no indication whether any given supplement contains video records (and if so how many) or, instead, such other ancillary material as still figures, additional tables, or protocol detail. Thus, the casual browser must engage in a bit of optimal foraging through the index linkages to find what he or she wants, which requires no great effort for some unusually fine pickings.
APOPTOSIS
Cell death, as a genetically programmed event rather than an accidental event, fascinates many undergraduates, irrespective of their career goals. Possibly the most visually striking movie I have seen of apoptosis appeared in an article by Morgan et al. in the June 19, 2002, issue (10.1083/jcb. 200204039; see Figure 1 for a still image). The behavior of HeLa cells transfected with a plasmid containing a chimeric gene for yellow fluorescent protein and the “death domain” of the receptor for tumor necrosis factor (YFP-TRADD-DD) was recorded during a 24-h period at a rate of one image every half-hour by using fluorescence and phase-contrast microscopy. The fluorescent and phase-contrast images at each time point were subsequently merged, and the published QuickTime movie contains 36 frames and lasts about 12 s. Thus, the sequence of portrayed events has been speeded up about 5400 times, and the results are generally well resolved and contrasted and are cinematically spectacular. The fluorescent chimera are seen localized in the HeLa nuclei, and, with time and despite occasional changes in focus, the movie clearly documents their aggregation as the nuclei of cells entering apoptosis become condensed. Following such condensation, the cells relatively quickly enter the final stages of apoptosis and begin to bleb along their peripheries. Curiously, fluorescence disappears in some cells just before they enter the blebbing stage, and with the benefit of hindsight I found myself wishing that images had been collected more frequently following nuclear condensation.
The authors note the absence of nuclear localization and apoptosis in cells transfected with the YFP gene alone, but no video record is provided of this control. It is unclear how apoptosis resulting from the truncated form of TRADD is related to that generated by the membrane-bound form of the receptor, as the authors note. Nevertheless, the movie is a visually arresting document and, in its brevity, a great lecture anthem on apoptosis for either an introductory or a nonscience audience. It may be found at http://www.jcb.org/cgi/content/full/jcb.200204039/DC1/1.
Figure 1. Apoptosis of HeLa cells, as shown in Video 1 of Morgan et al. (2002; http://www.jcb.org/cgi/content/full/jcb.200204039/DC1/1). (Reproduced from The Journal of Cell Biology, 2002, vol. 157, pp. 975-984, by copyright permission of The Rockefeller University Press.)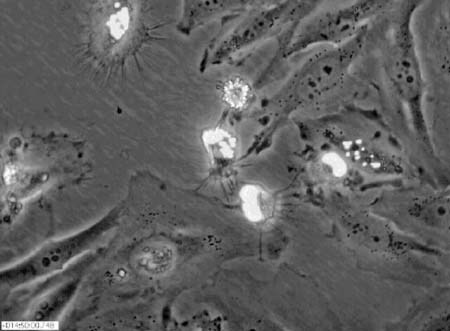
MEMBRANE LIPID DIFFUSION
For color overlays and technical refinement, my vote for the Best Video(s) of Issue goes to those accompanying an article by Fujiwara et al. (2002) describing the hop diffusion of an unsaturated phospholipid analogue (DOPE) within the plane of the plasma membranes of cultured normal rat kidney (NRK) fibroblasts (10.1083/jcb.200202050). It has been clear for more than a decade that integral membrane proteins (IMPs) are restricted in their diffusion within the plane of a cell membrane by peripheral membrane “corrals” of cytoskeletal elements or by transient “tethers” to these elements (see, for example, earlier work from the same laboratory: Tomishige and Kusumi, 1999; PMID:10436005). Using high-speed video microscopy and both fluorescent and gold-tagged DOPE, Fujiwara et al. present five temporally well-resolved movies indicating that membrane phospholipids are similarly restricted. Video 1 (see Figure 2A for a still image) depicts the diffusion of single, fluorescent, and gold-tagged DOPE within an NRK membrane, in two separate sequences, with 33-ms resolution. Video 2 (see Figure 2B for a still image) displays the more rapid behavior evident at higher temporal resolution (25μ s) in two sequences: first, that of a single, gold-tagged DOPE, which is then repeated in a second sequence with an overlay of the particle trajectories colored differentially to illustrate the transient restriction of diffusion to 230-nm corrals or compartments. Video 3 illustrates the control behavior of DOPE diffusing without restriction within the bilayer of a phospholipid liposome, again with a colored trajectory overlay. Video 4 presents overlaid images that suggest that phospholipid diffusion is also restricted by larger, 750-nm compartments, whereas Video 5 illustrates the effects of latrunculin-A and jasplakinolide, which are, respectively, actin destabilizing and stabilizing drugs, on the size of the 750-nm compartment and on the phospholipid residency period. Video clips 1, 3, and 5 may be found at http://www.jcb.org/cgi/content/full/jcb.200202050/DC1/3, ... /DC1/5, and ... /DC1/7, respectively.
Figure 2. (A) Hop diffusion of gold-tagged DOPE over 230-nm compartments observed at a 25-μs resolution for 62 ms (270 slowed), as shown in Video 2 of Fujiwara et al. (2002; http://www.jcb.org/cgi/content/full/jcb.200202050/DC1/4). (B) Hop diffusion of gold-tagged DOPE over 230-nm compartments observed at a 25-μs resolution for 62 ms (×270 slowed) with their trajectories superimposed, as shown in Video 2 of Fujiware et al. (2002; http://www.jcb.org/cgi/content/full/jcb.200202050/DC1/4). (A and B reproduced from The Journal of Cell Biology, 2002, vol. 157, pp. 1071-1082, by copyright permission of The Rockefeller University Press.)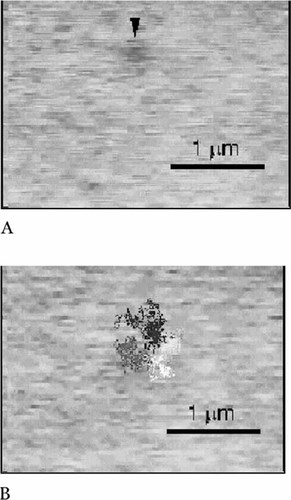
The Fujiwara et al. video records are so clear and well described in their captions that, with some background preparation, they could be used for introductory courses in cell biology during an experimental session in a computer laboratory. Alternatively, along with the article, they provide the basis for an extended discussion of “membrane concepts: facts and artifacts” in an advanced journal seminar. Using the records, students could manually record residency times and estimate compartment sizes from the color overlay sequences in Video clips 2 ( http://www.jcb.org/cgi/content/full/jcb.200202050/DC1/4) and 4 ( http://www.jcb.org/cgi/content/full/jcb.200202050/DC1/6). They could then compare these data with similar measurements of phospholipid diffusion in a pure phospholipid bilayer (from Video 3). Following this initial analysis, the possible effects of various cytoskeletal destabilizing and stabilizing drugs could be discussed and hypotheses formulated. The latter could then be tested by using the data in Video 5. (Alternatively, the effects of similar drugs might be presented and discussed and then students might be asked to determine from the data in Video 5 the functions of the two drugs used for the experiment.) Observant students examining these records might note in Video 4 the apparent stability of a larger compartment as the particle being tracked first diffuses out of and then back into the same membrane region. Moreover, a more thoughtful student might wonder whether the“ unrestricted movement” described for the singly colored trajectory presented in the control video (Video 3) might also appear more restricted and somewhat corralled if multiple colors had been used judiciously in constructing the overlay. In considering these and other questions, advanced students would necessarily want to consult the clearly written and well-documented article and especially the statistical analyses. These are excellent videos and a well-written article for undergraduate use at the intermediate and advanced levels and for graduate students.
MITOSIS AND EARLY DEVELOPMENT
Looking for videos of mitosis or of early developmental events following fertilization? Nice, clear, and well-resolved video movies of paired differential interference contrast (DIC) and fluorescent images of dividing Caenorhabditis elegans eggs were published by Hannak et al. (2002; 10:1083/jcb.200202047), accompanying their investigation of the importance of γ-tubulin in centrosome formation. Chimeric proteins containing green fluorescent protein (GFP) linked to γ-tubulin and to histone were expressed in eggs and sperm and used to follow the behavior, respectively, of centrosomes and chromosomes following fertilization. The paired images were all captured at either 6- or 8-s intervals, and because the movies are shown at 10 frames per second, the events depicted are speeded up 60- to 80-fold. Video 1 ( http://www.jcb.org/cgi/content/full/jcb.200202047/DC1/1; see Figure 3 for paired still images) depicts early nematode development during the first 13 min or so. All the crucial events are clearly evident in one or the other of the paired movies: pronuclear migration and syngamy (fusion), establishment of the mitotic poles, chromatin condensation into chromosomes, mitosis, and cytokinesis and the formation of a two-celled embryo. Because γ-tubulin is localized in centrosomes and centrosomes are contributed exclusively by sperm in animal development, only one of the two pronuclei in the fertilized egg is accompanied by a pair of brightly GFP-labeled centrosomes. Watching the behavior of this organelle and the orderly progression of mitosis, introductory students may wonder whether the centrosome exercises a choreographic role in mitosis. They may also question how the chromosomes become aligned during metaphase and separate during anaphase. To address these questions, they can view Video 3 ( http://www.jcb.org/cgi/content/full/jcb.200202047/DC1/3; see Figure 4 for a still image), which shows an identical sequence in an embryo expressing GFP-α-tubulin and well-labeled astral and spindle microtubules, as well as unorganized centrosomal tubulin. Observant students will also note the periodic appearance of GFP-histone-labeled polar bodies at the egg periphery in the various videos. Some quibbles, however: the Video 3 clip I was able to view lacked a paired DIC movie. Also, because GFP was used to label both proteins, instructors will need to identify for introductory students the centrosome and chromatin as the loci of γ-tubulin and histone, respectively. In sum, Videos 1 and 3 nicely illustrate the basic events of mitosis and early animal development and are eminently suitable for an introductory audience.
Figure 3. Wild-type embryo expressing GFP-histone and GFP-γ-tubulin, as shown in Video 1 of Hannak et al. (2002; http://www.jcb.org/cgi/content/full/jcb.200202047/DC1/1). (Reproduced from The Journal of Cell Biology, 2002, vol. 157, pp. 591-602, by copyright permission of The Rockefeller University Press.)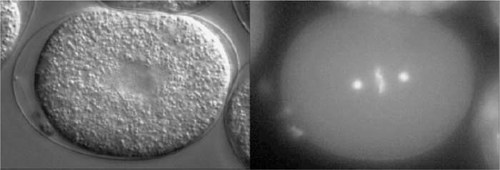
Figure 4. Wild-type embryo expressing GFP-α-tubulin, as shown in Video 3 of Hannak et al. (2002; http://www.jcb.org/cgi/content/full/jcb.200202047/DC1/3). (Reproduced from The Journal of Cell Biology, 2002, vol. 157, pp. 591-602, by copyright permission of The Rockefeller University Press.)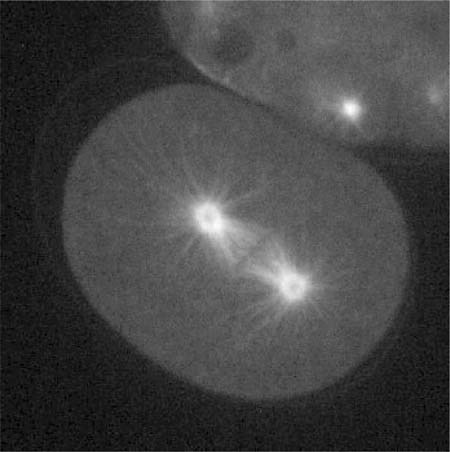
More advanced undergraduates and their instructors may want to explore the function of γ-tubulin in greater depth. If so, they will want to grapple with the figures and data of the Hannak et al. article and their use of RNAi as an experimental tool. Video 2 ( http://www.jcb.org/cgi/content/full/jcb.200202047/DC1/2) provides an excellent entry for such an exploration because it depicts the striking effect of an RNAi (against gip-1) that prevents the expression of a protein (CeGrip-1), which, in turn, is crucial for the centrosomal localization of γ-tubulin. Video 4 ( http://www.jcb.org/cgi/content/full/jcb.200202047/DC1/4) would also prove useful in this regard because it illustrates the effect of an RNAi for tbg-1 (the C. elegans γ-tubulin gene) on the organization of α-tubulin during early development.
ERRATUM
Finally, I want to revise some comments in my last review (Watters, 2002) about the arrangement of videos in the Molecular Biology of the Cell (MBC) paper by Sharpless and Harris (2002; 10.1091/mbc01.07.0356). Certain of the criticisms I voiced then concerning the confusing manner in which video clips were associated with unrelated figures have since been addressed. The clips are now linked with the appropriate figures and, in certain instances, renumbered as well. Movie 1 is now linked with Figure 5 and illustrates the localization of an actin-scaffolding protein called SEPA in a cytokinetic ring. Movie 3, which illustrates the location of SEPA at the tip of a growing hypha, has inexplicably been renumbered “2,” but its identification has not been changed in either the caption or the text. Four other movies—5 and 7 and 6 and 8—have been linked appropriately with Figures 7A and 7B, respectively, and they show the effects of cytochalasin A on SEPA dynamics in the contractile ring and the control data (Figure 7A) and in the hyphal tip also with control data (Figure 7B). Clip 2 seems to have been deleted and Clip 4 can now be located, without caption, through the link (MBC Video) that appears with the article title in the Table of Contents. These changes greatly improve the ease with which the videos can be accessed and, in turn, understood in context.
Again, I invite your comments on these reviews and your suggestions of other peer-reviewed videos for possible review as educational material.



