Video Views and Reviews
I could subtitle this feature “Kate's Cool Video Clips” because the three Molecular Biology of the Cell (MBC) movies I review here were suggested by Kate Durda, a biology major at Colby College in Maine, who is compiling an index of MBC videos for ASCB.
On a technical note, many of the video records I review in these features were obtained by “time-lapse photography”: that is, the images were captured at a slow rate (1 image s–1 or min–1 or h–1, for example), not at the usual rate of 16 or 24 images s–1. Most playback formats (such as QuickTime), however, project images at a standard rate of 24 s–1, and consequently, videos are usually speeded up with respect to the rate at which the events they record actually happened. The effect is quite dramatic and arresting but also artifactual! Thus, a movie containing 240 images may last 10 s, but if the events it portrays lasted 240 min (and were recorded at a rate of 1 image min–1), viewers will observe those events as if they were speeded up “1440-fold” or “1440 times.” I use this terminology to indicate how much the video records I review telescope the temporal events they record, but unfortunately, it is not always possible to obtain this information from the articles.
Figure 1. Figure 3 of Chytilova et al. (2000; http://www.molbiolcell.org/cgi/content/full/11/8/2733). Reproduced from Molecular Biology of the Cell, 2000, Vol. 11, pp. 2733–2741, by copyright permission of the American Society for Cell Biology.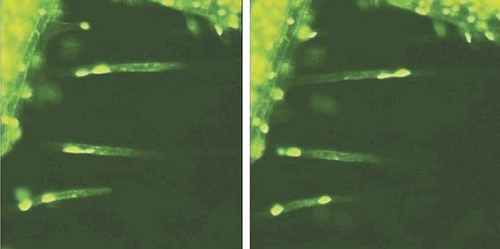
NUCLEAR DYNAMICS AND NUCLEAR LOCALIZATION SIGNALS
Videos that introductory-level students will likely find impressive accompany a paper on nuclear dynamics in Arabidopsis by Chytilova et al. (2000). Using confocal fluorescence microscopy, the authors recorded the movements of root hair nuclei labeled with a chimeric protein consisting of green fluorescent protein (GFP), a nuclear localization signal (NLS) and β-glucuronidase (GUS) from Escherichia coli. As demonstrated in a vivid time-lapse movie (their Figure 3; speeded up∼ 480-fold), nuclei are readily seen moving within root hairs, changing their shapes, and even separating into small bodies (which, however, remain connected by slender thread-like extensions, as illustrated in the paired images in Figure 1). No student seeing these dramatic movies will ever again think of nuclei as static structures! Their Table 1 documents the effects of several inhibitors of cytoskeletal organization, which indicate the nuclear movements are due to microfilaments and not microtubules, but unfortunately these results are not also presented as video records. Equally impressive, and possibly more suitable for intermediate cell biology students, is the authors' video record of the movement of the chimeric protein as a root hair nucleus passes through mitosis (Chytilova et al., 2000, Fig 6; speeded up about 235 times). Students must locate in the video the nucleus encircled in the still image, which requires careful observation. Tracking this nucleus during the video is complicated by the microscopic field of view gradually shifting in a diagonal manner from the upper right toward the lower left, which creates the somewhat disconcerting illusion that all the nuclei are “drifting upward” toward the upper-right corner. Even very attentive students may need to view this video several times. This extra effort will prove worthwhile, however, when they note the rapid dispersal of fluorescence (speeded up about 250-fold) as the designated nucleus (Figure 2; left-hand image) completes prophase of mitosis and its envelope breaks down (middle image). Then, several minutes later, during telophase, the GFP chimera may be seen slowly reaccumulating within the smaller daughter nuclei (right-hand image). The sequence presents a striking demonstration of the importance of the NLS, and it would be even more instructive were it paired with a control sequence documenting the behavior of a GFP:GUS chimera lacking the NLS.
Figure 2. Figure 6 of Chytilova et al. (2000; http://www.molbiolcell.org/cgi/content/full/11/8/2733). Reproduced from Molecular Biology of the Cell, 2000, Vol. 11, pp. 2733–2741, by copyright permission of the American Society for Cell Biology.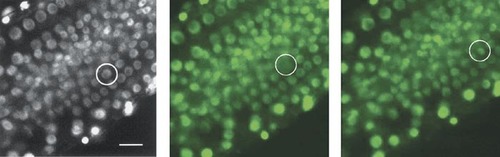
SPINDLE AND CHROMOSOMAL MOVEMENTS DURING MITOSIS
Those readers who missed the video review in the last issue of CBE, concerning γ -tubulin, mitosis, and early development in Caenorhabditis elegans (Hannak et al., 2002), may be interested in a similar paper published in MBC by Strome et al. (2001). In the latter paper, the authors present two sets of video fluorescence records concerning, respectively, the wild-type behavior of GFP:γ -tubulin and of GFP:β-tubulin during early cleavage. These records of control data are supplemented by additional videos that depict the consequence of γ -tubulin depletion (by RNAi) on the spindle organization of GFP:β-tubulin and on the mitotic behavior of chromosomes labeled with GFP:histone. Although the fluorescent images are not paired with differential interference contrast images, as is the case in the more recent study by Hannak et al. (2002), the results are nonetheless quite impressive, as illustrated in Figure 3. The upper images (Figure 3, A and B) illustrate the organization of β-tubulin at approximately metaphase during a normal mitosis (A) and at a similar stage in an (RNAi) embryonic cell lacking γ -tubulin (B). Note, in particular, the presence of a complete mitotic apparatus consisting of astral and both interpolar and chromosomal microtubules in the control embryo and of only astral microtubules surrounding two, poorly separated centrosomes in the RNAi-treated embryo. The lower images (Figure 3, C and D) illustrate a similar effect of the same RNAi on chromosomal orientation during mitosis, as inferred from the presence and condensed pattern of GFP:histone. The image of the control preparation (C) shows two cells in different stages of mitosis: the nearly invisible nucleus at the upper left is in metaphase, while the chromosomes in the nucleus at the lower right are just beginning to condense. In contrast, as illustrated in D, RNA interference with γ -tubulin production allows chromosomal condensation but completely eliminates the formation of a metaphase plate (or, indeed, any subsequent chromosomal separation during anaphase). With appropriate guidance, introductory students can follow the normal behavior of spindle and chromosomes in the control videos and can synthesize the separate images into an accurate composite picture of mitotic dynamics. More advanced students will likely enjoy speculating with the authors concerning the action of γ -tubulin on microtubule nucleation and subsequent function.
Moreover, the papers by Strome et al. (2001) and Hannak et al. (2002) provide excellent examples of the use of RNAi, and a detailed comparison and analysis of the two seem suitable for a graduate-level or an advanced undergraduate seminar course or for a journal club discussion. In such a context, the RNAi methodology could be examined in appropriate depth, and any difference in results discussed with reference to differences in the RNAi employed in the respective studies.
FIBROBLAST MOTILITY AND SUBSTRATE ADHESIVENESS
An actively moving cell continually attaches to the substratum along its leading edge and then detaches at its trailing edge in a fairly regular manner. If these changes in adhesiveness did not occur, a cell would exhibit intracellular vesicular movement; additionally, it might seem to oscillate or become elongated and greatly attenuated. But it wouldn't move! While focusing on the gymnastics and mechanisms of myosin–actin interactions, students (and their teachers) often overlook this crucial, anchoring aspect of cell motility, and I was therefore pleased to see the recent MBC paper on adhesiveness and fibroblast locomotion by Munevar et al. (2001).
Figure 3. Figures A and B are taken from videos that accompany Strome et al. (2001; http://www.molbiolcell.org/cgi/content/full/12/6/1751) Figure 8, videos N2 GFPbetaB.mov and SevRNAi GFPbeta.mov respectively. Figures C and D are taken from Strome et al., Figure 9, videos N2 GFPhist.mov and SevRNAi GFPhist.mov. Reproduced from Molecular Biology of the Cell, 2001, Vol. 12, pp. 1751–1764, by copyright permission of the American Society for Cell Biology.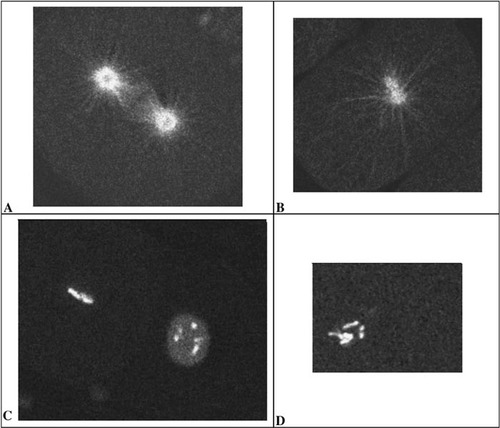
Despite Durda's recommendation, I almost passed over reviewing the paired videos accompanying this article, because half of the images are quite complex and were produced by a process—“traction force microscopy”—likely to be unfamiliar to many CBE readers and confusing to most students. Moreover, all video records are appended to figures in the article without discussion or caption descriptions, leaving the reader to infer what is happening in the movies from the more piecemeal information accompanying the still images.
As I replayed the videos, however, I became fascinated by the manner in which the paired videos depict locomotion: namely, as phase contrast images of a moving fibroblast alongside “traction force” images of the substrate distortion created by the moving cell. I was equally intrigued (and, in my ignorance, uncritically so) by the use of fluorescent beads embedded in a thin, distortable polyacrylamide substratum (containing Type I collagen) to gauge the traction force exerted by moving fibroblasts. As a cell moves, it distorts the substratum in proportion to its locomotion and to the strength of its adhesions. An algorithm using the relative distance each bead moved from its resting location estimated the tension of each distortion, and an image of these distortions was pseudocolored to show a range from low (purple) to high (red) intensity. Thus, for each of the four locomotive sequences recorded on video, the viewer sees (on the left) a phase contrast image of a fibroblast and, simultaneously (on the right), an image of the distorted substratum beneath the fibroblast. While it is difficult to discern how intracellular events generate the tensions that distort the substratum, or how locomotion would appear on a substratum not as distortable as polyacrylamide, merely watching a fibroblast move in concert with its pseudocolored doppelgänger is impressive. And as the two still images in Figure 4 suggest, the movies seemed sufficiently interesting and pedagogically useful to warrant review and recommendation to CBE readers.
I also think intermediate-level students will readily appreciate the use of an artificial peptide containing a binding motif to inhibit competitively integrin binding to collagen: Its effects are predictable, quite dramatic, and very well documented in two of the movies. Unfortunately, the possible relationship of intracellular actin-based events with the traction forces is only cursorily discussed, and advanced students will likely want to explore the interplay of actin assembly and treadmilling, vesicular transport and endo- and exocytosis, and membrane anchoring by reference to Bray's (2001) monograph on cell motility or the more recent cell biology text by Pollard and Earnshaw (2002).
Figure 4. Still images from Munevar et al. (2001; http://www.molbiolcell.org/cgi/content/full/12/12/3947). Reproduced from Molecular Biology of the Cell, 2001, Vol. 12, pp. 3497–3954, by copyright permission of the American Society for Cell Biology.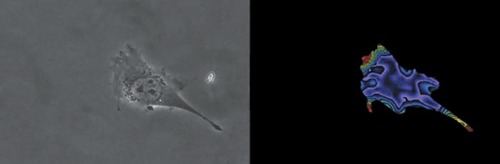
ANGIOGENESIS (THE DEVELOPMENT OF BLOOD VESSELS)
Often we are so caught up studying subcellular mechanisms that we lose sight of the fact that most eukaryotic cells are specialized components of tissues, organs, and organisms. As such, much cellular behavior is understandable only in histological or larger contexts. Students, however, usually come at cellular biology from these more familiar, macroscopic vantages, and it is always useful when teaching undergraduates to be able to situate cellular events in their broader settings. I was delighted, therefore, to read a recent article in the Tube Morphogenesis Series of Trends in Cell Biology that contains a brief, but interesting video clip of the growth and differentiation of a blood vessel, during the process called angiogenesis (Weinstein, 2002). The phenomenon was recorded from the forebrain of a transparent zebrafish embryo whose vasculature had been made fluorescent through the expression of a transfected plasmid containing a GFP gene fused with a promoter specific for vascular endothelium. The URL for the clip is http://archive.bmn.com/supp/tcb/movie3.html.
Figure 5. Still image from Weinstein (2002). Reproduced from Trends in Cell Biology, 2002, Vol. 12, pp. 439–445, by copyright permission of Elsevier Science.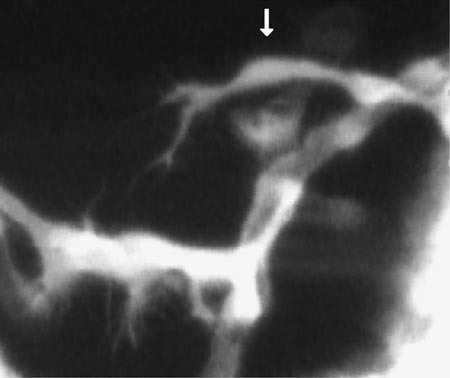
In Figure 5, taken from the video, an endothelial cell is shown arching its way across the top of the image and down toward a larger cerebral artery, with which it will form a communicating vessel (in a process that takes about 8 h). In the video, numerous filopodial extensions are readily evident extending (and retracting) both from the leading edge of the growing cell and from cells of the target artery. Interestingly, when contact is made between endothelial cell and target artery, filopodial activity ceases. Students with some knowledge of embryonic development will immediately recognize the similarity of these“ searching”filopods to the pathfinding behavior of archenteron cells during sea urchin gastrulation or of axonal growth cones during vertebrate neurogenesis, and they might well speculate about common mechanisms involving attractive and repulsive, diffusible and substratum cues. These discussions might lead, for example, to consideration of such plasma membrane proteins as Ephs, which are tyrosine kinase receptors, and ephrins, which are lipid-linked proteins that are Eph ligands. Questions might also arise as to how possible Eph and ephrin involvement in pathfinding could be tested and to the consequences within target and pathfinding cells of this ligand–receptor interaction. Students might also want to investigate what is known of the GFP promoter (for Fli1, a transcription factor) and why it is also expressed during embryogenesis in neural crest and certain myeloid derivatives. Moreover, instructors interested in producing fluorescent embryos for other vascular investigations, or simply for general demonstration purposes, will be interested in the well-illustrated and well-organized article and in the description of the more advanced “molecular epistasis” experiments. All in all, this article is a good read.



