Video Views and Reviews
INTRODUCTION
These days, all introductory biology students know that sliding filaments of myosin and actin cause muscle contraction. Moreover, cell biology students learn that there are more ubiquitous, less specialized forms of cell motility than what is evident in contracting muscle cells. Increasingly, it seems that actin is involved in a wider variety of motility phenomena than can be accounted for simply by the sliding movement of stable filaments. These phenomena rely not only on different types of myosins but on a different property of actin, namely, its highly regulated assembly and disassembly (e.g., Becker et al., 2002; see also, Watters, 2002).
The sliding and assembly/disassembly paradigms are explored in the first set of videos in this essay. The second set focuses on the subtle but crucial importance of cell attachment in all forms of locomotion and specifically on the role of integral membrane proteins in mediating the attachment of assembling and sliding filaments in the cytoplasm with the extracellular substratum.
I have not yet discussed any of these articles with undergraduates, and should readers chose to use them with their students, I would appreciate hearing any comments that arise from the discussions. As always, I also welcome readers' suggestions of other peer-reviewed papers containing video records they consider suitable for educational usage [[email protected]].
ACTIN AND THE GLIDING BEHAVIOR OF PLASMODIAL PARASITES
I very much enjoyed wrestling with an article published earlier this year by Wetzel et al. (2003), concerning the roles of myosin and of actin polymerization in the “gliding” motility exhibited by the intracellular parasite, Toxoplasma gondii. The video records accompanying this article are not complete, but fortunately, the paper referenced an earlier article from the same laboratory (Hakansson et al., 1999). Unlike many research articles, which seem to attach video records to their electronically archived papers as afterthoughts, the 1999 paper described several videos of the gliding behavior of T. gondi at length; these were obtained by time-lapse video microscopy. Viewing the 1999 videos was crucial to appreciating the later study. Taken together, the two papers present a good introduction to the peculiar motility of a group of parasites that were once considered sporozoans (protozoans) but are now members of a separate phylum of unicellular organisms, the Apicomplexa.
Why bother studying the gliding behavior of an obscure group of“ protozoans”? I readily admit to being easily caught up in the intellectual exercise of examining paradigms in light of what some would consider exotica, because paradigms require testing, and the better a paradigm is, the more phenomena it explains. Also, it's fun to examine with students an unfamiliar phenomenon or a genuinely open question, to test our understanding of basic concepts. We discuss what we know and then create testable hypotheses to explain what we don't understand. I was also drawn to these papers because many years ago I published my first Abstract on the gliding behavior of marine gregarines, organisms that are related to T. gondii (Watters, 1962). Toxiplasma is more closely related to the malarial-causing Plasmodium, and for the sake of medical relevance it can be argued that a basic understanding of gliding motility could provide a clue to effective malarial prophylaxis.
Toxoplasma and other Apicomplexae are thought to move through tissues and invade host cells by gliding, unaided by such organelles as pili, cilia, or flagellae. In vitro, however, the organisms exhibited several different forms of motility, including both circular and helical gliding and a kind of twirling (Hakansson et al., 1999). All three forms are well documented by video records, and the helical gliding motion is illustrated in Figure 1. Helical gliding seems more closely related to the motility exhibited by the parasites in escaping from a host cell and moving to invade an adjacent, uninfected cell. [Readers should view the impressive video of an invasive event, which accompanies their Fig. 7 ( http://www.molbiolcell.org/content/vol10/issue11/images/data/3539/F7/DC1/figure7.mov).] Thus, the motility exhibited seemed related to the manner in which the cells are attached to their environment. The authors also documented the importance of secreted or shed lipids and proteins as locomotory “trails.” Inhibitors of actin filament aggregation (cytochalasin D) and myosin (butanedione monoximine) demonstrated the importance actin–myosin interaction in generating all three forms of motility.
Although the images are obscured somewhat by the “halos” usually produced by phase contrast, most introductory cell biology students should find these video records, as well as the 1999 article, very accessible. A useful student discussion could be focused on explaining how the various forms of motility exhibited in vitro might reflect differences in the cells' interactions with their environment (e.g., how the cells attach to and detach from the substratum). Also, students might want to speculate on how the underlying sliding filament mechanism could generate all three forms of motility, how the parasite actually invades its host cell, and how the motility is regulated. They might also want to learn more about the possible regulatory role of intracellular calcium, in both host cell and parasite, for example. The authors' model of helical gliding would be especially useful for these investigations (Hakansson et al., 1999, Fig. 8), but students would probably want more information about the ultrastructural organization of actin microfilaments, especially the polarity of the filaments and their possibly helical or spiral orientation.
Figure 1. The helical gliding motion of T. gondii is depicted in three stills (A, B, and C) abstracted from a video record about 1 s apart. The parasite is approximately 7 μm long and is moving in a corkscrew manner from left to right ( http://www.molbiolcell.org/content/vol10/issue11/images/data/3539/F5/DC1/figure5.mov).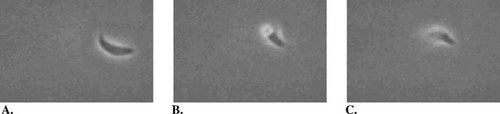
More inquisitive students may also want to know whether actin assembly and disassembly are involved in gliding, as they are in cells that move by lamellipodial ruffling (see Small et al., 2002). This possibility has been addressed more recently in a study published by the same laboratory, using jasplakinolide (JAS) to stabilize actin in its filamentous, or F-actin, form (Wetzel et al., 2003). Apparently, F-actin is very unstable in T. gondii: Only small quantities of the filamentous form are detected by standard biochemical or electron microscopical methods, and these results suggest that most of the actin is in its monomeric, or G-actin, form.
As expected, JAS treatment increased the amount of F-actin, and it produced spiral arrays of filaments in the cortex. The stabilizing agent's effect on the three forms of motility usually observed in vitro was, in contrast, somewhat paradoxical. Circular gliding was never observed in any of the treated specimens. Although helical movements reminiscent of those seen during helical gliding were observed, treated cells so frequently reversed their direction of twisting that they failed to progress across the substratum. While the gliding behavior of untreated cells was described, readers were referred to the earlier study for video documentation of this behavior (Hakansson et al., 1999). A critical student might wonder whether the substance used to solubilize JAS (DMSO) would also affect motility and, consequently, would question whether the behavior documented in the 1999 videos, in the absence of DMSO, provides a sufficient control for the JAS effects described by Wetzel et al. (2003). Twirling was also observed, but at a surprisingly accelerated rate (Figure 2). During the 2.3 s interval separating Figure 2, A and B, the untreated specimen rotated in a clockwise direction approximately one revolution; during the 13.8 video the cell rotated ca. six times (for a velocity of about 0.43 rps). In the presence of JAS (Figure 2, C and D), cells rotated at a rate of approximately 0.80 rps, or almost twice as fast as the control. Both rates lie within the highly variable range of values reported in their Table 1. Twirling in JAS-treated cells frequently changed direction, from clockwise to counterclockwise and back.
Students will be hard-pressed to interpret these results, as were the authors, and students should be encouraged to review the 1999 paper and any prior discussion of how the three forms of motility in the various video records might be related. Then important areas of uncertainly could be pinpointed and examined more critically. Given the data, it seems reasonable to agree with the authors that the presence of F-actin is rate-limiting as far as motility is concerned. Understanding the direction of contraction and, indeed, how that direction changes with JAS treatment will require more information about the orientation of F-actin, and myosin behavior, than is available in these papers. In this regard, it is interesting that“ cortical stripping” of untreated cells produced parallel arrays of filaments in the exposed cell surface strips. Similarily, JAS treatment produced spiral patterns of actin immunofluorescence beneath the cell surface. Given these data, it is easy to imagine how spiral orientation of F-actin could generate torsional movements by a myosinactin sliding mechanism, if the actin filaments all possessed the same polarity with respect to sliding myosin. Unfortunately, no such arrays were found in the cortical strips removed from JAS-treated cells and the actin immunofluorescence of untreated cells seemed diffuse and neither filamentous nor cortically localized. Further discussion along these lines, including possible methodological problems and more extensive consideration of the relevant literature (e.g., Menard, 2001), would be more suitable for graduate or advanced undergraduate seminars or journal club sessions.
Figure 2. A single rotation of a tethered T. gondii under control conditions (A and B; from http://www.molbiolcell.org/content/vol0/issue2003/images/data/E02-08-0458/DC1/Video1pc.mov) and in the presence of 2 μM jasplakinolide (JAS) (C and D; from http://www.molbiolcell.org/content/vol0/issue2003/images/data/E02-08-0458/DC1/Video2pc.mov). The inset scales indicate elapsed time from 0.000 s.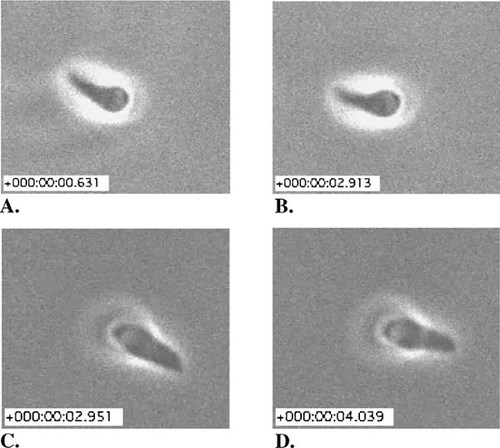
Considered sequentially, these two papers present an excellent opportunity for students to appreciate the nature of scientific investigation and the ways in which experimental manipulation provides answers, ambiguities, and often myriad new questions. The first paper reviewed here—there are earlier ones from this laboratory on Toxoplasma motility—presents essentially a series of interesting observations of gliding behavior that are well documented with video records and related experimentally to the sliding filament paradigm for cell motility. The second paper explores the possible importance of actin assembly/disassembly in gliding behavior, but the results presented seem less complete and more ambiguous, perhaps reflecting the novelty of our experience with the heuristic value of macromolecular assembly and disassembly.
THE ROLE OF INTEGRINS IN REGULATING LAMELLIPODIAL LOCOMOTION
As their name implies, integrins are integral membrane proteins (IMP). Some integrins form clusters in the plasma membrane of animal cells, and these clusters (called focal adhesions) attach the cells to their substrata composed of extracellular matrix (ECM) proteins and glycoproteins. Integrins not only anchor cells to the ECM but also nucleate the assembly of cytoskeletal elements (such as filamentous forms of actin) and mediate transmembrane signals that lead to cytoskeletal reorganization and cell motility (see, e.g., Lauffenburger and Horwitz, 1996). These IMP are actually heterodimers; that is, they consist of two dissimilar, α and β subunits (which in turn are identified numerically, as in“ α6β4”). Currently, 18 types of α subunits and 8 types of β subunits have been found in vertebrate cells, and the list is growing (see, e.g., Lodish et al., 2003).
Figure 3. Still images from videos of cell migration in wound-healing assays, showing, from left to right, the behavior of CHO cells and CHO cells expressing cDNA for either α4 or α4:GFP chimera. Video1: http://www.molbiolcell.org/content/vol13/issue9/images/data/3203/F1/DC1/Video1.mov. Video 2. http://www.molbiolcell.org/content/vol13/issue9/images/data/3203/F1/DC1/Video2.mov. Video 3: http://www.molbiolcell.org/content/vol13/issue9/images/data/3203/F1/DC1/Video3.mov.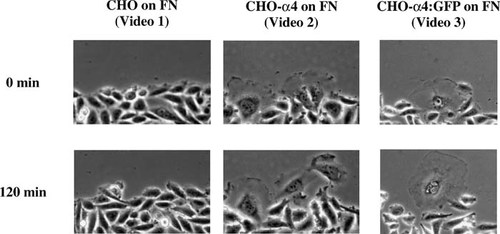
Not all integrins form focal adhesions, however: α4β1, for example, is usually absent from such attachments, while α5β1 forms junctional complexes. The role of these two integrins in affecting the motility of Chinese hamster ovary (CHO) cells has been explored recently by Pinco et al. (2002). Their results seem straightforward and are especially well documented by 10 video records. Although these records are untitled and are not accompanied by captions, the text explicitly associates each with particular figure stills. Moreover, the videos show the behavior of cell populations, and both the images and the article should be readily accessible to intermediate-level cell biology students. Introductory students should also appreciate the earlier videos in the series with minimal guidance from an instructor. The events were recorded under time-lapse conditions and are speeded up 60-fold in the QuickTime movies: that is, any change that occurred over a minute appears with a duration of 1 s.
To assess the role of α4β1 in motility, the authors used an in vitro wound-healing assay in which CHO cells were grown to confluence on fibronectin (FN). The confluent cell layer was scraped to produce a narrow cell-free zone with two free edges, and the rate of cell outgrowth from one edge and individual cell morphology were recorded by phase-contrast or fluorescence microscopy. Since CHO cells express α5β1 but not α4β1, the behavior of cells transfected with plasmids containing cDNA for either α4 or a chimera linking α4 with green fluorescent protein (α4:GFP) was also assessed, with results illustrated in Fig. 3. After 120 min, the control cells showed little tendency to migrate into the“ wound” zone, while cells expressing α4, and presumably α4β1 (with or without GFP), flattened, spread, and exhibited distinctive, ruffling lamellipodia and outward migratory behavior at the wound edges. Video records were also made of cells ectopically expressing α4 and grown on VCAM-1 (a specific ligand for α4β1) or on a recombinant peptide containing RGD (the tripeptide portion of FN specific for α5β1). The records nicely demonstrate ruffling and outward migration on VCAM-1 and very little change in CHO morphology (reminiscent of control behavior) on RGD.
Thus, the first five video records very nicely illustrate the rather clear-cut dependence of lamellipodial migration of CHO cells on the presence of integrin α4β1. Observant students will likely note that the videos of cells transfected with α4 alone show slightly greater lamellipodial activity than those transfected with the GFP chimera, suggesting an inhibitory effect of the fluorescent peptide on locomotion. While the quantitative data presented by the authors in their Figure 3 indicate that the GFP chimera slightly stimulated the formation of lamellipodia, it might be worthwhile for students to discuss how such chimeras might affect the outcome of experiments and produce artifacts, as the authors examined at length. More advanced students, for example, might well be encouraged to draw the α4:GFP plasmid and identify where the chimeric cDNA attaches the fluorescent peptide to α4. They then might consider how such a construct could conceivably alter the role of α4β1 in intracellular signaling and/or cytoskeletal attachment. In this regard, they would find the video record accompanying Figure 5 in the article especially useful: http://www.molbiolcell.org/content/vol13/issue9/images/data/3203/F5/DC1/Video9.mov.
The article (and remaining video records) also describe the wound-healing in CHO mutants that express negligible levels of α5β1 or fail to express paxillin, a signaling and adaptor protein that binds to the cytoplasmic tail of α4. Consideration of these data would round out a journal club presentation or an advanced seminar class devoted to the importance of the α4 subunit of integrin in facilitating lamellipodial formation and motility.



