Video Views and Reviews: Golgi Export, Targeting, and Plasma Membrane Caveolae
In this essay, I review videos from Molecular Biology of the Cell (MBC) depicting various aspects of plasma membrane (PM) dynamics, including the targeting of newly synthesized components and the organization of those PM invaginations called caveolae. The papers accompanying these videos describe, respectively, the constitutive export of a mutant form of the vesicular stromatitis virus coat glycoprotein (VSVG) from the Golgi complex (Polishchuk et al., 2003); the receptor-mediated, PM incorporation of the Na+/K+-pump (Bertorello et al., 2003); and the cell surface organization of caveolae (Thorn et al., 2003).
As is customary, I appreciate hearing your reactions to these reviews and your suggestions of other peer-reviewed videos for possible review as educational material.
CONSTITUTIVE EXPORT AND TRANS-GOLGI TUBULAR NETWORK (TGN) DYNAMICS
Green fluorescence protein (GFP) is now a well-established marker for following in living cells the pathway taken by newly synthesized protein to which GFP has been attached, and video observations of this pathway and of its experimental perturbations are numerous in the literature (see Lippincott-Schwartz et al., 2000; also, Watters, 2002). Recently, Polishchuk and associates (2003) monitored the transport of Golgi-derived vesicles containing newly synthesized viral coat protein in several cultured cell lines, which they transfected with the hybrid gene for GFP-VSVG. Twelve video records are archived with their article and are likely to be of interest to Cell Biology Education readers for several reasons. First, for the science: the marker apparently moves by bulk flow in a selective, three-stage and kinesin-mediated process along microtubules from the trans-Golgi network (TGN) to the PM. To demonstrate these features, the authors perturbed vesicular formation at the TGN, analyzed the presence of various vesicular components immunocytochemically at light and electron microscopic levels, and noted that VSVG and a larger cargo protein (pro-collagen 1; PC-1) followed the same pathway.
All but two of the 12 videos document different experiments, and each is keyed to a different figure in the paper, as illustrated in Table 1. Movies 1a and 1b illustrate the formation of vesicles following a double temperature shift (from 40°C to 20°C to 32°C), which was the “standard protocol” for subsequent experiments, and Movie 2 illustrates the recovery of vesicle formation following a partial bleaching of the Golgi. Figure 1 shows three images taken from Movie 1a.
| Movie | Figure | Treatment: cell lineb |
|---|---|---|
| 1a | 1A | Recovery following double temperature shift: Cos7.c |
| 1b | 1B | Recovery following double temperature shift: Cos7.c |
| 2 | 2 | Vesicle formation following partial photo-bleaching: Cos7. |
| 3a | 3A | Control for tubulin localization assay: Cos7. |
| 3b | 3B–D | Control for kinesin localization assay: Cos7. |
| 3ca | 3E | Control for TGN46 protein localization assay: Cos7. |
| 3da | 3E | Control for TGN46 protein localization assay: Cos7. |
| 5 | 5A–B | Experiment showing effect of anti-NSF antibody: BHK. |
| 6 | 6E–H | Control for VSVG and PC-1 localization assay: HF. |
| 7 | 7A | Control for TEM localization of vesicle formation: Cos7. |
| 8 | 8A | Control for TEM localization of vesicle formation: HF. |
| 9 | ? | Unidentified cell bearing red and green fluorescence. |
HONORING GEORGE PALADE ON THE OCCASION OF HIS NINETY-SECOND BIRTHDAY
This essay is dedicated to George Palade, Nobel Laureate, founding member and past president of ASCB, and, most recently, dean emeritus of the UCSD School of Medicine, on the occasion this November of his 92nd birthday. The videos and research papers reviewed here reflect, in a small way, the rich legacy of research provided by Palade and his associates over the last half of the 20th century. The first two sets of videos demonstrate different aspects of the vectoral pathway taken by newly synthesized plasma membrane proteins (see Jamison and Palade, 1977). The caveolae video is of special interest because the structures themselves were discovered in transverse serial sections of vascular endothelia cells by Palade (1953).
Also, the videos are interesting and I think especially useful for teaching purposes because, except for Movie 5, all depict control cells prior to experimental treatment or fixation and further processing. For all treatments, and prior to fixation, the behavior of GFP-VSVG in individual cells was recorded by video fluorescence microscopy (thus producing for each an “internal” control for what followed). After fixation, each cell was processed immunocytochemically for light or electron microscopy, and the result was recorded and presented in an authors' figure, with notable exceptions. (Rather annoyingly, and contrary to confusing text entries, Movies 3c and 3d are identical, while the cell depicted in Movie 9 is not the cell depicted in authors' Figure 9 and is otherwise unidentified.)
Presentation of such repetitious videos seems boring and unnecessary—but only in the misleading sense that control data appear boring and unnecessary. Annoyance (and poor editing) aside, what makes these video records interesting pedagogically is that they make up data sets of replicated, transfected, but otherwise unmanipulated cells: that is, seven different Cos7 cells, two different HFs, and one BHK. They provide students with an opportunity to observe multiple cells from the same line, and cells from different lines, under similar conditions—and not just a single image of a “representative cell.” I would consider using these videos with a class of intermediate-level biology students broken up into “lab” groups of two or three seated around computer workstations or laptops. Following a PowerPoint or comparable introduction to GFP technology (e.g., transfection and fluorescence microscopy), I would then work with the students through Movie 1a and ask them to describe and briefly discuss what they see. Many will not recognize the Golgi complex when comparing it with the mental images they have formed from textbook figures, much less appreciate the Medusa-like writhing of the TGN (which also would be sufficient reason for showing the videos to an introductory class of students). Then I would ask each group to examine one or several other movies and again describe what they see, gradually building in the ensuing discussion a more general picture of similarities and differences in vesicle behavior within and among the different cell cultures. Short of students actually working with these cells in culture and making and discussing their own videos, I can think of no better way of introducing them to the pleomorphic features of the Golgi complex and the vesicular traffic surrounding it.
Figure 1. Consecutive fluorescence images taken of a Cos7 cell transfected with GFP-VSVG over 5 sec: at 0.0 sec (A), 2.8 sec (B) and 4.9 sec (C). An extension of the TGN projects toward “11 o'clock” and small arrowheads indicate sites of vesiculation from this extension. Subsequent fixation and immunocytochemistry indicate the extension is oriented along microtubules. The video may be found at http://www.molbiolcell.org/content/vol0/issue2003/images/data/E03-01-0033/DC1/movie_3a.mov.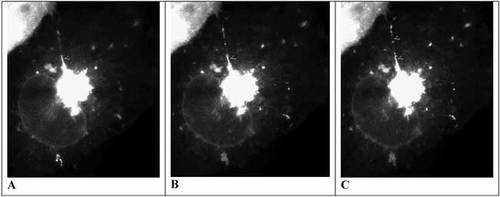
Critical examination of the control videos begs numerous questions, and inquisitive students are sure to raise them, such as: how are the TGN tubules extended, how do the vesicles form, and how are vesicles directed toward the PM? To partially address these questions, hypotheses could be tendered, Movies 3a and 3b could be reviewed, and data from authors' Figure 3A and 3B-D could be considered concerning the colocalization of GFP-VSVG and, respectively, tubulin and kinesin. Several other questions will arise in similar fashion (concerning, for example, the importance of various vesiculation cofactors such as the proteins β-COP and NSF) and lead to an examination of other Figures (such as 4 and 5). Thus, another reason why I find these videos to be useful instructional instruments is that they present inviting and relatively easy portals for students to gain access to the article itself. After we've read the title of an article and noted the lab of origin (and possibly scanned the abstract), most scientists enter research articles by looking first at the figures and tables. Although exciting, jumping into articles in this fashion requires a certain level of sophistication, and I believe the videos archived by Polishchuck et al. allow even inexperienced students a similar experience.
Figure 2. Fluorescent images of an A549 cell transfected with GFP-Nkα1 (the α-subunit of the Na+/K+-pump) at lower magnification (A) and at higher magnification, approximately 54 sec (B) and 2 min 10 sec (C) after dopamine stimulation. The arrows indicate two vesicles about to fuse with the PM. The videos may be found at http://www.molbiolcell.org/cgi/content/full/E02-06-0367/DC1.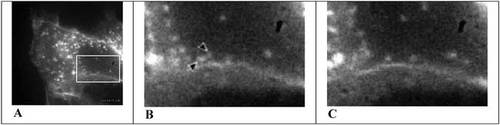
Finally, advanced students will want to dissect the explanatory model the authors present in their Figure 10 and consider whether it does justice to the data sets they have carefully examined. The very critical may well wonder to what extent the pleomorphism they observe might be an artifactual result of transfection with an efficient plasmid and even whether viral infection itself might cause similar results in host cells in vivo.
A provocative but accessible and “readable” article!
RECEPTOR-MEDIATED INCORPORATION OF THE NA+/K+-PUMP INTO THE PM
Generally speaking, the activity of ATP-dependent ion pumps, like that of other enzymes, can be modulated in two ways: directly, through active site-related and allosteric mechanisms; and indirectly, through changes in pump number and location. In the latter instance, and like other transport proteins (notably an isoform of the glucose transporter, GLUT4: see Lodish et al., 2003), storage vesicles containing cryptic Na+/K+-pumps fuse with the PM in response to physiological need. Specifically, in lung alveolar cells, Na+/K+-pump recruitment appears to be stimulated through a dopamine-sensitive, G-protein regulated pathway (Ridge et al., 2002). To investigate the mechanism of this regulation, Bertorello et al. (2003) transfected the N-terminal fragment of the catalytic subunit of the pump (NKα) tagged with GFP into cultured human lung carcinoma cells (A549). They characterized the basal and dopamine-stimulated behavior of storage vesicles containing the tagged proteins with fluorescence microscopy under various experimental conditions. Two video records of the effects of dopamine on fluorescent vesicle behavior are archived with their article, depicting low- and high-magnification images captured at 1-sec intervals and corresponding to authors' Figure 1. Three frames from these videos are presented in Figure 2.
I recommend these videos and the research article for intermediate and advanced undergraduates for the precision of observation and thought their scrutiny encourages. Not surprisingly, untreated cells have active pumps and, correspondingly, their images in these videos exhibit substantial fluorescence in both vesicles and the PM. Thus, any stimulatory effect of dopamine might well be visually subtle. After explaining what the images represent and describing, briefly, the biochemical evidence for dopamine's action (Ridge et al., 2002), I would ask students to view the low-magnification video several times, describe vesicular behavior, and then characterize any effect of dopamine on that behavior. At first viewing, and even after several reruns, they will likely infer particle behavior is unaffected by dopamine, as indeed I did (although fluorescence of the PM does increase incrementally for short periods). Dopamine does increase the rate of pumping activity, however, by about a third (authors' Figure 1B), and if this increment results from a corresponding increase in pump number, then vesicle behavior should be changing in some manner. Questions then could be raised about how vesicle behavior in a fluid can be characterized more precisely, quantitatively rather than qualitatively, and how Brownian movement of vesicles might change if they become associated with cytoskeletal elements. This discussion then naturally leads to scrutiny, respectively, of authors' Figure 2 and, for tests of cytoskeletal involvement, of authors' Figures 3, 4, and 5. Critical students, noting that PM pump activity is actually a steady-state phenomenon, might wonder whether some increment in activity might also result from a reduction in the number of pumps being removed from the PM.
I also would encourage students to view the higher-magnification video several times, first at “normal speed” and then frame by frame (by pressing the >> arrow on the QT control bar). The fusion events that are obvious at normal speed may seem obscure when viewed one frame at a time. Good discussion can be developed around the artifactual nature of time-lapse photography, the mind's tendency to interpolate across temporal gaps, and the inherent uncertainty of our knowledge when events of msec duration, such as vesicle movement and fusion, are “recorded” with video images at 1-sec intervals.
GLOBAL PATTERNS OF ADIPOCYTE CAVEOLAE
Beginning with a pioneering study by Palade (1953; see also Fawcett, 1966), animal cell surfaces have been known to contain small, flask-shaped invaginations called caveolae. Originally hypothesized to be involved in“ micropinocytosis” and in transcytosis of molecules across endothelial cells, these invaginations are now thought to engage in signal transduction as well. Caveolae membranes are especially enriched in cholesterol and the invaginations are present in many different cell types (see, e.g., Alberts et al., 2002).
Figure 3. Scanning electron microscope images organized as a video, showing first at lower magnification, a field of adipocytes (A), and at increasing magnification, their outer plasma membrane surfaces (B and C). In the latter, darker spots indicate caveolar orifices. Later in the simulated video, a single adipocyte (D) is hemi-sectioned, illustrating a protruding nucleus, the central fat droplet, and the inner surface of the PM (colored green). Increasingly higher magnification produces images of the caveolar indentations at the inner surface as illustrated in E and F. The video may be found at http://www.molbiolcell.org/content/vol0/issue2003/images/data/E03-01-0050/DC1/Video1.mov.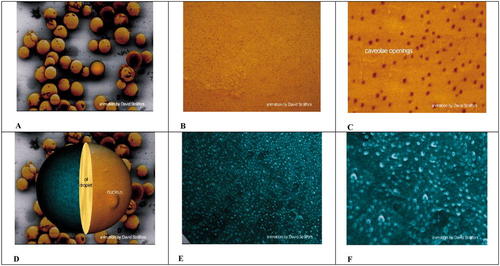
Thorn et al. (2003) extend our knowledge of caveolae through their scanning electron microscope (SEM) studies of both the outer and inner surfaces of adipocyte PM, and their video represents an appropriate visualization for illustrating these features to introductory students. The video differs significantly from the type usually reviewed in this column, however, in that it was constructed from a series of scanning electron micrographs of the outside and inside surfaces of the plasma membranes of fixed cells and not obtained from living cells. The sequence portrayed is thus an animation of still images that depict spatial and not temporal changes.
Figure 3 presents six images from the animation, and Figures 3C and 3E are especially interesting. They represent images of caveolae at the extracellular and intracellular surfaces of adipocyte plasma membranes, respectively, and most students will immediately recognize differences in caveolae number in the two images. They may also notice a difference in the sizes of vesicles attached to the intracellular PM surface. Discussion of what the numerical difference represents and whether it is meaningfully correlated with the size difference provides a natural entry to the paper itself and, specifically, to the hypothesis that larger caveolae are open to the surface while smaller ones are closed (and simply tethered to the surface) and to how this hypothesis was tested.
Questions are raised easily about how the caveolae are formed and whether this process resembles how clathrin-coated vesicles are formed. Apparently, a coating protein called caveolin is involved in the process, but its presence is restricted to caveolae necks: the authors' Figure 5 depicts a single clathrin-coated vesicle (with its honeycomb morphology) and several smoother caveolae. The authors estimate that open-ended caveolae increase the effective surface area of adipocytes significantly, and thoughtful students will speculate about the functional significance of such an increment (and, indeed, about the function of both populations of caveolae). Consideration of these broader questions could well become the focus of a journal club presentation (see Parton, 2001).



