Advantages and Challenges of Using Physics Curricula as a Model for Reforming an Undergraduate Biology Course
Abstract
We report on the development of a life sciences curriculum, targeted to undergraduate students, which was modeled after a commercially available physics curriculum and based on aspects of how people learn. Our paper describes the collaborative development process and necessary modifications required to apply a physics pedagogical model in a life sciences context. While some approaches were easily adapted, others provided significant challenges. Among these challenges were: representations of energy, introducing definitions, the placement of Scientists’ Ideas, and the replicability of data. In modifying the curriculum to address these challenges, we have come to see them as speaking to deeper differences between the disciplines, namely that introductory physics—for example, Newton's laws, magnetism, light—is a science of pairwise interaction, while introductory biology—for example, photosynthesis, evolution, cycling of matter in ecosystems—is a science of linked processes, and we suggest that this is how the two disciplines are presented in introductory classes. We illustrate this tension through an analysis of our adaptations of the physics curriculum for instruction on the cycling of matter and energy; we show that modifications of the physics curriculum to address the biological framework promotes strong gains in student understanding of these topics, as evidenced by analysis of student work.
INTRODUCTION
In 1910, the educator and philosopher John Dewey noted that “science teaching has suffered because science has been so frequently presented just as so much ready-made knowledge, so much subject-matter of fact and law, rather than as the effective method of inquiry into any subject-matter” (cited in Archambault, 1964, p. 182). Nearly 100 yr later, this sentiment that science is best learned through active inquiry and exploration rather than a traditional lecture approach is echoed by the reports and recommendations of the American Association for the Advancement of Science (AAAS, 1997, 2011), the National Science Foundation (NSF, 1996), and the National Research Council (NRC; Bransford et al., 1999). Unfortunately, the available textbooks and curricula commonly stress memorization over the deep conceptual understanding that develops when students engage in inquiry-based activities and discussions that help them construct their own understanding of science concepts.
Reform in these areas is particularly critical when considering the training of our nation's future teachers. Teachers model their own teaching after the classroom experiences they had as learners more than on the theory or even the classroom experiences they encounter in teacher education programs (Grossman, 1991). Unfortunately, the contrast between what is expected of future teachers in their K–12 classrooms and what they experience in content and instruction in typical college or university science courses can be quite striking (Darling-Hammond and Bransford, 2005). Thus, a targeted approach to teacher education must begin in their undergraduate science preparation, and prospective teachers should be taught science in a manner that replicates the inquiry strategies and active learning that we hope they will employ in their own classrooms (McDermott, 2006). Many elementary teachers, even experienced ones, are uncomfortable teaching science for a variety of reasons, including poor scientific literacy, negative attitudes toward science, and the belief that science is difficult to teach (see van Aalderen-Smeets et al. [2012] and references therein). Because elementary teachers feel the least prepared to teach science compared with other subjects (Fulp, 2002; Dorph et al., 2007), it is crucial that any undergraduate science classes they do experience employ the inquiry strategies and constructivist approaches that characterize best practices in science education. Indeed, modeling effective inquiry strategies can change the practice of elementary teachers, causing them to use more inquiry in their own classrooms (Staples, 2002).
Preparing preservice elementary teachers to teach science effectively was one important aspect of the North Cascades and Olympic Science Partnership (NCOSP), an NSF-funded Math–Science Partnership housed at Western Washington University (WWU). One way we addressed this was by designing a year-long sequence of three science courses (in physical science, life sciences, and earth science) targeted to elementary education students completing their credentials at WWU but open to all undergraduates. Initially, we sought published science curricula that adopted a student-centered, constructivist, active, and inquiry-based approach to deepen science content knowledge in a manner appropriate for elementary education undergraduates.
More than other science disciplines, the physics education community has developed curricula that meet these criteria, including Physics by Inquiry (McDermott, 1996), Modeling Instruction (Hestenes, 1987; http://modelinginstruction.org), Investigative Science Learning Environments (Etkina and Van Heuvelen, 2007; Etkina et al., 2010), and Physics for Elementary Teachers (now called Physics and Everyday Thinking [PET], Goldberg et al., 2005). Although all of these curricula were developed based on research on how people learn, they differ in their pedagogical approaches and in their target audiences. Of the curricula available at the time, PET best suited our needs for the physics course of our three-course sequence, because its pedagogy fit our class structure (small, laboratory-based classes) and its target audience is elementary teachers. However, as we began preparing for the other science courses in the sequence (those focused on life and earth sciences), we were faced with a lack of cohesive, constructivist curricula appropriate for our needs. In life sciences, although there are several examples of reformed introductory courses, these tend to focus on implementing innovative instructional strategies into large classes (e.g., Friedrichsen, 2001; Lawson et al., 2002; Chaplin and Manske, 2005; Hoskins et al., 2007; Uekert et al., 2011). We required materials for a smaller, lab-based course that modeled pedagogy that could be used by preservice elementary teachers. Thus, to provide a cohesive experience for students taking all three classes, we decided to develop a life sciences curriculum using PET as a model. (An earth science class using PET as a model was also developed by other faculty associated with NCOSP.)
As a discipline, biology presents some unique challenges to student learning. Recent work in the field of developmental cognitive psychology has elucidated why some common misconceptions persist, given how the human mind functions. Coley and Tanner (2012) sum these up as “cognitive construals,” which they define as “informal, intuitive ways of thinking about the world.” Cognitive construals are ways in which people process, understand, and make decisions about information, and some cognitive construals have the capability to influence how a student learns biology. The authors argue that three are particularly important to biology education. The first is teleological thinking, a type of causal reasoning in which students assign a goal or purpose to a process. The common misconception that plants produce oxygen because animals need it is an example of teleological thinking. This type of thinking is common and useful in everyday thought, because it allows us to interpret events and behaviors; it is therefore persistent even when contradictory evidence is available (Kelemen, 1999). The second cognitive construal identified by Coley and Tanner (2012) as particularly troublesome for learning biology is essentialist thinking, in which students assign an essential property, or “essence,” to members of a group or category along with other summary information about that group. For instance, many students consider DNA to be the essential property that makes a cell a cell. Such thinking can lead to misconceptions, such as thinking that cells in a body must have different DNA, because the cells look different from one another. Essentialist thinking can also lead a learner to believe that there is little or no variation in a group. Finally, the third cognitive construal that is important to biology education is anthropocentric thinking. In this case, students use analogies about humans to try to make sense of unfamiliar biological phenomena or processes. The common misconception that plants take in food molecules through their roots is an example of this type of thinking.
Students also have difficulty understanding concepts at the different scales (atomic, cellular, organismal, ecosystem) that apply to biological phenomena. Hartley et al. (2011) argue that this is partly due to the pervasiveness of informal reasoning (reasoning that uses simple “actors” that cause events to happen in the presence of “enablers”) as opposed to principle-based reasoning, which relies on using fundamental laws to explain phenomena. To fully understand many concepts in biology, students must be able to use principle-based reasoning to move across scales. For example, to understand how energy and matter move through living systems, students must be able to use the laws of conservation of energy and matter to move from the atomic to the ecosystem level (Wilson et al., 2006; Hartley et al., 2011).
Overcoming these challenges will require a change in how biology is taught to undergraduates, and there have been several recent reports calling for such a change (for an overview, see Labov et al., 2010). In particular, Vision and Change in Undergraduate Biology: A Call to Action (AAAS, 2011) outlines best practices in teaching biology, practices that are still rare in most undergraduate biology classrooms, in which lecture is the norm, and content is covered at a rapid rate. The authors of Vision and Change advocate covering fewer concepts in greater depth, making learning goals for core concepts explicit to students, and integrating science process skills throughout the curriculum. They also advocate “student-centered learning,” which involves students as active learners in all classes, uses different types of instruction (including lecture), and integrates multiple forms of ongoing assessment throughout a course. These changes are necessary, especially given the rapid advances in biology research and the increasing intersection of biology with other disciplines.
In this paper, we describe the process we used to develop a reformed life sciences curriculum that uses a physics curriculum model, addresses the challenges inherent to learning biology, and responds to the call to change how undergraduate biology is taught. We briefly describe the resulting materials and then discuss the challenges we faced in adapting a life sciences curriculum based on a physics model. Finally, we illustrate the effectiveness of our curriculum, using a well-established, open-ended assessment that was given to students in several different courses, including some not using our curriculum.
CURRICULUM DESIGN AND DEVELOPMENT
The life sciences curriculum, called Life Science and Everyday Thinking (LSET), was initially developed, beginning in September 2004, by a group of higher-education faculty from a regional university (WWU), three local community colleges that provide the majority of transfer students to WWU (Everett Community College, Skagit Valley College, and Whatcom Community College), and the Northwest Indian College. The initial curriculum was intended for a 10-wk course and was subsequently revised, beginning in February 2010, to a 16-wk curriculum for institutions on the semester system. This last revision was done in collaboration with colleagues at California State University, Chico (CSU, Chico) and with middle- and high-school teachers from the Bellingham, Washington, area.
Our target audience was preservice elementary teachers, although we wanted the curriculum to be appropriate for all nonscience majors, as many of the students taking the courses at the community colleges did not intend to become teachers. WWU has a large teacher preparation program and, in the past, our preservice elementary teachers took a minimal number of “introductory” science courses from a broad menu of topics and disciplines. These courses were often survey courses that provided a superficial treatment of many topics within a discipline, and lecturing was the predominant instructional strategy used. Thus, our elementary education students were not being provided the opportunity to develop deep, conceptual understanding of relevant content, nor were they able to observe instruction that applied the recommendations of research findings on how people learn. Our students now take a three-course sequence that includes PET, LSET, and the earth science curriculum also developed through NCOSP. (A fourth course on chemistry has just been developed.) CSU, Chico also has a large population of future teachers who previously had little coherent science preparation targeted to their needs—the development of the semester-long version of LSET was a response to meet those needs.
To link the courses in the sequence, LSET explicitly uses the same pedagogy as PET. We also linked the courses thematically, by using the flow of matter and energy in living systems as focal points for LSET. Overall, LSET covers the one-way flow of energy from sunlight captured by plants though the trophic levels, with heat loss throughout. These concepts are contrasted with the cyclical flow of matter, focusing on carbon. Over five chapters, the curriculum presents these concepts at the cellular, organismal, and ecosystem levels. It also has two chapters (one on genetics and one on evolution) less directly related to this main theme that we considered necessary to include in the curriculum due to elementary teacher preparation guidelines in several states. The full curriculum can be covered in a semester. On the quarter system, instructors have the choice of two coherent paths: one focusing on the organismal/ecosystem concepts and one focusing on the organismal/cellular concepts. Figure 1 illustrates the range of concepts covered by LSET.
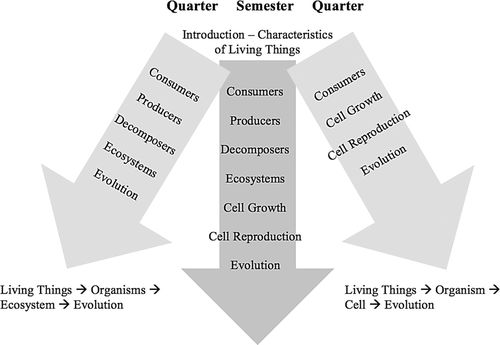
Figure 1. Topics covered by LSET in a semester-long or quarter-long course. There are two coherent paths that instructors on the quarter system can follow.
A “backward design” (Wiggins and McTighe, 1998) was used to develop the curriculum. Thus, we first identified what we wanted our students to know or be able to do at the end of a chapter and developed assessment items around these learning goals. Then we developed the chapter to better enable students to construct understanding toward those goals.
Figure 2 provides a conceptual model of the specific steps we used during the development process. The process began with identifying the key concepts (we refer to them as “big ideas”) that students need in order to understand the topics covered in the course. Content experts from the university and community colleges consulted the state and national science standards in circulation at the time of curriculum development, including the Grade Level Expectations for Washington State Essential Academic Learning Requirements (Washington State Office of the Superintendent of Public Instruction [WSOSPI], 2005), the National Science Education Standards (NRC, 1996), and the Benchmarks for Science Literacy and Atlas of Science Literacy (AAAS, 1993, 2000) for identification of these big ideas. They also relied on their own training. For example, two of the big ideas found in chapter 4 of LSET (“How do matter and energy cycle in living systems?”) are:
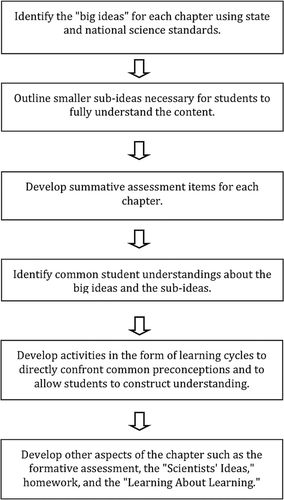
Figure 2. Conceptual model of the “backward design” process used to develop LSET.
Energy flows through an ecosystem, entering mostly as light, passing through as chemical energy in organic compounds, and exiting as heat; and
Matter, in the form of essential chemical elements, is recycled within an ecosystem by decomposers, which decompose organic material and return elements to reservoirs.
These big ideas are echoed in the National Science Education Standards (NRC, 1996), which state the by the end of high school students should understand that:
As matter and energy flows through different levels of organization of living systems—cells, organs, organisms, communities—and between living systems and the physical environment, chemical elements are recombined in different ways. Each recombination results in storage and dissipation of energy into the environment as heat. Matter and energy are conserved in each change. (pp. 186–187)
The Benchmarks also incorporate this idea, but over a range of age groups. By middle school, students should understand that:
Over a long time, matter is transferred from one organism to another repeatedly and between organisms and their physical environment. As in all material systems, the total amount of matter remains constant, even though its form and location change.
Energy can change from one form to another in living things. (p. 120)
and by the end of high school they should understand how these concepts apply to populations and ecosystems:
At times, environmental conditions are such that land and marine organisms reproduce and grow faster than they die and decompose to simple carbon containing molecules that are returned to the environment. Over time, layers of energy-rich organic material inside the earth have been chemically changed into great coal beds and oil pools.
The chemical elements that make up the molecules of living things pass through food webs and are combined and recombined in different ways. At each link in a food web, some energy is stored in newly made structures but much is dissipated into the environment. Continual input of energy from sunlight keeps the process going. (p. 121)
This big idea is also found in state standards documents. The Washington State Essential Academic Learning Requirements (WSOSPI, 2005) state that by the end of high school students should understand that
Matter cycles and energy flows through living and nonliving components in ecosystems. The transfer of matter and energy is important for maintaining the health and sustainability of an ecosystem. (p. 98)
Finally, this big idea is found in one of the core concepts for biological literacy outlined in the recently released Vision and Change in Undergraduate Biology: A Call to Action (AAAS, 2011):
Biological systems grow and change by processes based upon chemical transformation pathways and are governed by the laws of thermodynamics. (p. 13)
This tight relationship between the LSET big ideas and the state and national science standards is found throughout the LSET curriculum.
Once the big ideas were established (a process that took several months), we used them to identify the content critical to understanding them. With this in mind, we outlined the smaller ideas, or “subideas,” necessary to construct understanding of the big ideas and how those ideas link together. Another set of ideas was developed that address the skills and practices inherent to the practice of science regardless of discipline, for example, experimental design, data presentation, and identification of patterns. Our goal was that no individual activities should address these “process” ideas on their own; rather, these skills and practices should be embedded within developing biology content, as part of what it means to do and learn science.
Once the big ideas were identified, the curriculum was divided into chapters and individual or pairs of faculty members agreed to work on a chapter. Summative assessments were written. Common initial student ideas were identified for each of the big ideas (AAAS, 1993; Driver et al., 1994), and the curriculum was developed to specifically expose and confront these preconceptions.
For the curriculum, we relied on commonly used experiments, but we linked them together in a manner that reflects how students best learn science. Rather than having students complete experiments in a “cookbook” manner, each chapter was subdivided into activities, and each activity was developed to follow a learning cycle. For example, students are led to “discover” that plants undergo both photosynthesis and cellular respiration by first recording their initial ideas about what will happen to oxygen and carbon dioxide levels in closed chambers containing plants when the chambers are in the light and in the dark. They discuss their initial ideas with one another and then present their ideas to the class using a whiteboard. They then measure oxygen and carbon dioxide levels and record their data in their notebooks and on a class graph. Throughout the experiment, they answer questions that deliberately link their results to earlier measurements of gas levels in chambers containing animals (the concept of cellular respiration was initially developed in a previous chapter). In the next experiment, they observe infrared pictures of a corpse flower to conclude that plants give off heat, again answering questions that link their observations to the products of cellular respiration. At the end of the activity, students revisit their initial ideas and document how their thinking has changed. Thus, rather than just following a procedure and recording data, students’ preconceptions are elicited, conceptual understanding is constructed, and students engage in metacognition at the end of the activity. This learning cycle is repeated for each activity within a chapter.
The structure of a chapter is illustrated in Figure 3, which uses chapter 3 (“What Is Food for Plants?”) as an example of how the different components within a chapter are organized. Briefly, at the beginning of each chapter, the purpose is stated, and the students are prompted to complete a formative assessment that addresses the big ideas of the chapter. They discuss their ideas in small groups and then share those ideas with the class using whiteboards. This allows instructors and all class participants to hear the variety of initial ideas. Students then complete a series of activities designed to specifically address common ideas and to allow students to construct knowledge in a sequential manner, as described above. In general, the experiments include laboratory activities, thought experiments, and exercises using paper and computer models. Throughout these experiments, students are required to make predictions, gather data, and draw conclusions. At the end of each activity, students are prompted to explicitly reconsider ideas held before the activity and to document any change in their thinking. At times when “telling” is required, material is presented as Scientists’ Ideas, in which students read factual information about the concepts they have been investigating and link their observations and results to that information. Homework is assigned after most activities, and it is done individually to allow instructors to check for individual student understanding of the key concepts from the activity. At the end of each chapter, students revisit the ideas they held in the formative assessment at the beginning of the chapter and consider how their thinking has changed. They also participate in a group discussion about the big ideas of the chapter, and this is a chance for the instructor to guide students to link concepts into a broader picture. Finally, each chapter contains a Learning about Learning activity, in which students consider different aspects of how people learn as they apply to the LSET curriculum and to elementary classrooms.
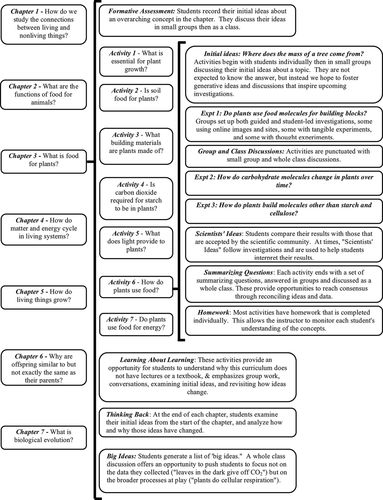
Figure 3. Overview of the LSET curriculum using chapter 3 as an example of the curriculum structure. Each chapter and each activity within the chapter are organized with the same learning cycle components.
Comparing the Physics and Biology Curricula
Table 1 compares the learning principles used in PET (Goldberg et al., 2005) with the learning principles in our curriculum. These principles are derived from research in cognitive science and science education and are not unique to physics; further details on their origins are described in Goldberg et al. (2010). It was relatively straightforward to incorporate these elements into the biology curriculum, as noted in the rightmost column of Table 1. There were occasional challenges in developing a good elicitation question, finding appropriate tools, and scaffolding skills, but these challenges did not seem unique to biology, suggesting (as expected) that these learning principles are relatively independent of scientific content.
| Learning principles | How PET applies principles | How LSET applies principles |
|---|---|---|
| Students’ prior knowledge influences their learning. | Student's initial ideas are elicited at the beginning of most activities. Activities make use of students’ intuitive ideas and build on previously constructed ideas. | As with PET, most activities begin with initial ideas. For example, students have an intuitive sense that there are no cells the size of a watermelon, that animals lose weight by excreting it, and that plants must take in solid materials to grow. |
| Students’ knowledge may be resistant and is often at odds with science ideas. | Activities are explicitly designed to elicit and then address commonly held ideas. Students are asked to revisit their initial ideas at the end of activities. | The ideas in LSET, as in PET, are explicitly elicited and engaged through the curriculum and revisited at the end of each chapter. |
| Students construct knowledge gradually in a complex process requiring multiple exposures. | Activities within and across chapters build on one another. Particularly resistant ideas are addressed explicitly several times in different contexts. | Not only does LSET build through the entire semester—tackling the theme of “what is life” and flows of matter and energy in living systems—but it builds on ideas developed in PET. |
| Complex skills can be scaffolded and modeled over time. | The skills of constructing and evaluating a scientific explanation are first introduced with a lot of support and structure. This support fades over the length of the course. | These skills—first introduced in PET—are continued in LSET, developing the ideas over two semesters (or quarters). In addition, exploring the flow of matter and energy is first introduced in relatively simple systems and builds to consider an entire ecosystem through this curriculum. |
| Students’ learning is mediated by social interactions. | Students engage in cooperative learning by working through the activities in small groups. The end of every activity also includes class discussion of some initial ideas and summarizing questions. | In LSET, as in PET, students work closely on investigations in small groups and then use whole-class discussions to discuss and evaluate the claims they can now make. |
| Interaction with tools is critical to learning. | Whenever possible, students perform hands-on experiments to gather evidence. Computer simulations, video, and instructor demonstrations extend this experience. | Tools, including CO2 detectors, thermometers, microscopes, whiteboards, online data, fossils, and those used in student-generated experiments, are central to the curriculum. |
Nonetheless, there were elements of the PET curriculum that required a significant rethinking. In particular, challenges included:
Using representations of energy developed for physics contexts (e.g., a diagram showing how a block gains and loses energy as it is pushed) in biological contexts (e.g., a diagram showing how a tree gains and loses energy);
Defining fundamental concepts (life, species, evolution);
Handling anomalous results; and,
Incorporating scientists’ ideas at the end of a chapter.
We have come to characterize these challenges as reflecting a difference between the core ideas presented in introductory physics and biology. In particular, we interpret these challenges through the following lens: introductory physics is often presented as a science of pairwise interactions; introductory biology is often taught as a science of linked processes. In introductory physics, pairwise interactions (e.g., the free body diagram and the PET energy transfer diagram) underlie explanations and predictions for phenomena. In introductory biology, understanding the types of core processes, including their inputs, outputs, and functions, is the building block upon which explanations of biological phenomena rest. And, we claim, this difference accounts for the primary challenges we faced in developing a biology curriculum, LSET, based on a pedagogy successful in a physics context, PET.
In the following sections, we offer support for these claims by examining the Framework for K–12 Science Education (NRC, 2012), LSET, and PET, using cellular respiration as a brief case study. It is worth noting that these orientations (pairwise vs. linked processes) are not necessarily intrinsic to the disciplines: expertise in biology often requires an understanding of the interactions underlying processes. Similarly, expertise in physics requires being able to move fluidly from simple pairwise interactions to aggregate phenomena.
Cellular Respiration as a Biological Process
Take, for example, a core topic in introductory biology: aerobic cellular respiration. In our view, the key biological idea of aerobic cellular respiration is that it is the process by which chemical energy, stored in the arrangement of food molecules (such as glucose), is transferred to ATP, allowing a living organism to use the energy in ATP to carry out the necessary processes of life. For this transfer to take place, the atoms of molecular oxygen and glucose are rearranged into water and carbon dioxide, which are then typically released from the cell. This process is one that connects producers, consumers, and their abiotic environment. It helps to explain such key observations and questions in biology as: Why do we need to breathe? What is the purpose of food? What kinds of substances give us energy? What kinds of organisms need oxygen? What role does breathing play in losing weight? How are organisms connected to one another and to their environment?
This description of the core ideas of cellular respiration is not an idiosyncrasy of the LSET curriculum, but is echoed in the new Framework for K–12 Science Education (NRC, 2012), which notes that (by grade 12) students should understand the following ideas related to matter and energy flows as part of the disciplinary core ideas for the life sciences:
Aerobic cellular respiration is a chemical process in which the bonds of food molecules and oxygen molecules are broken and new compounds are formed that can transport energy to muscles … Cellular respiration also releases the energy needed to maintain body temperature despite ongoing energy loss to the surrounding environment. (p. 148)
In both LSET and the Framework, the core idea of cellular respiration is a process, rather than an interaction, and our attention is drawn to characterizations of matter and energy at the beginnings and endpoints of processes, not the beginnings and endpoints of pairwise interactions. At the start of cellular respiration, there are certain forms of energy present in the arrangement of molecules; at the end of this process, the energy is located in different molecules. Other questions may arise: In what way do food and oxygen molecules “break,” form new molecules, and maintain temperature? Why should these interactions form new molecules that can “transport energy”? How does a molecule “transport energy”? Such questions are legitimate scientific questions—however, they are not presented as biological questions. Instead, they point toward the (usually pairwise) interactions that underlie biological processes and are not part of what is considered introductory biology content.
By way of comparison, consider the physical science disciplinary core ideas presented in the Framework that address cellular respiration (among other biological processes):
A variety of multistage physical and chemical processes in living organisms, particularly within their cells, account for the transport and transfer (release or uptake) of energy needed for life functions. (p. 130)
Such a statement is so vague as to be virtually useless as an explanation; instead, we view this description as an epistemological claim: students should understand that underlying the process of cellular respiration that biology claims as a core idea are a multitude of physical interactions that account for those biological phenomena. This core idea, then, regards the kind of explanations offered in the different disciplines: physical science is nested at a more fundamental level, attending to multistage interactions.
How, then, would physics address the questions of transfers of energy in cellular respiration?
Transfers of Energy as Pairwise Interactions
The PET curriculum addresses how to account for the changes in energy that take place during chemical reactions as the attractive and repulsive components of atoms rearrange during processes such as cellular respiration by guiding students to construct the following idea:
If the mutual interactions between the components of a system are attractive, then as the average distance between the components increases, the potential energy of the system increases also. If the mutual interactions between the components of a system are repulsive, then as the average distance between the components increases, the potential energy of the system decreases. (Goldberg et al., 2005, p. 3-89 in Scientists' Ideas)
And, again, this idea is not an idiosyncrasy of the curriculum, but is an idea that is echoed in the Framework. The physical science core disciplinary ideas that address how arrangements of atoms might store energy claims that:
When two objects interacting through a force field change relative position, the energy stored in the force field is changed. Each force between the two interacting objects acts in the direction such that motion in that direction would reduce the energy in the force field between the objects. (NRC, 2012, p. 127)
The two statements convey the same idea: One interaction between two objects leads to a predictable change in energy. These descriptions provide a mechanism by which two interacting objects gain or lose energy— addressing why a ball thrown upward slows down as it rises, or why attracting magnets accelerate as they come together. Using a PET approach in the LSET curriculum, then, we would describe both the food–oxygen pairing as the container of chemical potential energy and the attractive forces that convert chemical potential to kinetic energy as the food and oxygen are brought closer together.
It is reasonable to assume that the topics in introductory physics are also explained by more fundamental interactions than are presented in the typical introductory course—that is, much like introductory biology does not concern itself with the detailed, molecular mechanisms of the Krebs cycle to establish core ideas regarding the flow of matter and energy, there may be a multitude of mechanisms underlying the means by which two interacting objects change their energy that are not the part of the canon of introductory physics. However, unlike the Framework's statement acknowledging that physical processes underlie biological processes, there is no analogous statement in physics; the Framework does not claim, for example, that
A variety of multistage subatomic and quantum processes account for the transfer of energy between two interacting objects.
Though this is in fact true, introductory physics—at least within the Framework and PET —presents itself as being a base-level, fundamental mechanism for the sciences, dealing with interactions between two irreducible objects. (It is not that the objects are fundamentally irreducible, but that they are presented as such.)
Effects on Curriculum Development
During the development of LSET, the difference between processes and interactions manifested itself in the following ways:
Using representations to track the flow energy;
Defining fundamental concepts;
Handling anomalous results;
Incorporating scientists’ ideas.
In the following sections, we detail how the difference between interactions and processes presented challenges for these topics, how we addressed them in our curriculum, and the results on student understanding of the flow of matter and energy in ecosystems.
Representations to Track the Flow of Energy
PET and LSET both frame much of their instruction on characterizing and following the transfer and transformation of energy through systems, whether they are living systems (cells, organisms and ecosystems) or physical systems (colliding carts, interacting magnets, or heated gas). Because of this, we had hoped to use the energy diagrams from PET to characterize energy flows in living systems.
For example, a hand pushing a cart at constant speed along a surface might be represented as shown in Figure 4. This energy diagram depicts all of the relevant interacting objects by linking the objects with an arrow; for each interaction (labeled by an underline), there is a transfer of energy, described by the form of energy in the arrow. This transfer affects the amount of energy present in each object, as indicated in the bubble below the object; energy decreases in the source and increases in the receiver. (If two objects interact via more than one type of interaction, there would more than one arrow linking the objects.)
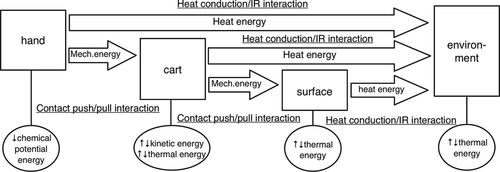
Figure 4. Energy diagram from PET for a hand pushing a cart at constant speed. The diagram tracks energy transfers and transformations through a sequence of pairwise interactions (e.g., contact push–pull, heat conduction, and infrared interactions) between objects.
When seeking to describe the discrete interactions through which energy transfers and transforms during, say, cellular respiration, the sheer number of interactions is problematic. Moreover, in conversations between biologists and physicists, we found that such moment-to-moment, fine-grained descriptions of energy transfers and transformations were not part of the basic canon of introductory biology. A physicist, for example, would find it problematic to claim that energy “in” the bonds of glucose is transferred to the bonds of ATP and would locate that energy instead in the broader glucose–oxygen system, and yet, rather than claiming that biology gets it wrong, we have come to interpret this as attending to a level of mechanism that is not addressed by introductory biology (for a more detailed discussion on chemical bonds and energy in biological systems, see Redish [2012]; for an analysis of how biologists and physicists define and describe these concepts differently, see Hartley et al. [2012]). That is, we do not wish to teach biology as physics, and therefore must modify the representational format to better capture the kinds of questions and answers that biologists offer when considering the flow of energy.
So instead of calling students’ attentions to the steps of glycolysis/Krebs cycle—the pairwise interactions between molecules—we modified these energy diagrams to describe significant biological processes. The representations still follow the source/receiver path for energy, but the arrows represent particular kinds of life processes, as shown in Figure 5, rather than interactions.
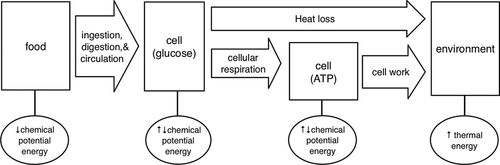
Figure 5. Energy diagram from LSET for food being used for energy. The diagram tracks energy through a series of linked processes (e.g., ingestion, cellular respiration, cell work) that transfer and transform energy.
Table 2 summarizes how the physics curriculum attends to particular pairwise interactions and how the biology curriculum attends to biological processes in tracking the flow of energy by describing a representative set of interactions (physics) and processes (biology).
| PET interactions and the effects on energy | LSET processes and the effects on energy | ||
|---|---|---|---|
| Contact push–pull interaction | Touching objects that push or pull on each other transfer mechanical energy and change an object's kinetic energy. | Photosynthesis | Using light energy from the sun, plants convert carbon dioxide and water to food molecules that have chemical potential energy. |
| Gravitational, magnetic, and electrical interactions | Objects with mass, magnetic/ferromagnetic objects, and charged objects that are near each other transfer mechanical energy and change an object's kinetic energy. | Ingestion, digestion, and circulation | Chemical potential energy is delivered to a cell by taking food molecules from outside the body, separating them into small pieces, and delivering them into a cell. |
| Light interaction | A source of light illuminates an object, transfers light energy from the source, and changes the thermal energy within the object. | (Aerobic) cellular respiration | Food molecules in the cell combine with oxygen (creating water and carbon dioxide), and energy from the food is transferred to ATP. |
| Heat conduction/infrared interaction | Objects at different temperatures transfer heat and cause a change in thermal energy in one or both objects. | Cell work | Energy in ATP is converted to kinetic energy so a cell can carry out necessary functions. |
Defining Fundamental Concepts
A second difference between the two curricula lies in the role that definitions play. Throughout the physics curriculum, core concepts—for example, kinetic energy, thermal energy, and mechanical energy—are defined for students. Within LSET, we chose to ask students to construct definitions for many key terms. For example, the term “food” is used without a strict definition when discussing animals, but when we begin to discuss what counts as food for a plant, students struggle with how to best define the term. Through explorations involving oxygen and carbon dioxide sensors, with plants in the light and plants in the dark, students consider how plants acquire the materials for growth and energy and use these ideas to construct their own definitions for food. For many classes, this results in a debate in which students argue whether or not carbon dioxide and sunlight should be considered food, or whether a material can only be considered food when the matter and energy are together in a single food molecule.
Within biology, determining what should be considered food is a question that ties to the role that food molecules play in ecosystems, organisms, and cells. It is only through understanding the role of certain molecules as building blocks and energy providers for all life processes that our definition of food makes sense. Defining terms such as “food,” “life,” and “species” requires an understanding of the broader processes in which these terms have their meaning (cellular respiration, ecosystems, and evolution). That is, many key definitions in biology are so tied to the processes in which they derive their meaning that defining the terms outside of the context of the process (and before students have investigated and understood the process) is problematic. What makes a producer a producer is its ability to create food from nonfood molecules through photosynthesis; what makes food “food” is its role in growth and cellular respiration. Absent understandings of these key processes (photosynthesis, growth, and cellular respiration), such definitions are meaningless, so constructing definitions goes hand-in-hand with inquiry into biological processes.
By way of comparison, PET begins the curriculum with the following definition (the second activity of the first chapter; the first activity familiarizes students with motion detectors and their graphs):
Scientists associate a form of energy with the motion of an object—they call it kinetic energy. The faster an object is moving, the more kinetic energy it possesses. (Of course, an object at rest possesses no kinetic energy.) Thus, as the speed of an object changes, the amount of kinetic energy it has also changes. (Goldberg et al., 2005, p. 1-21, emphasis in the original)
With this definition in place, students investigate which kinds of interactions with which kinds of objects cause changes in kinetic energy. The interactions are so spare—a single hand interacting with a cart on a track—that calling attention to one aspect of the interaction is not “giving away” a finding, nor does it require understanding the broader framework of energy to make sense of this definition. Thus, in PET, definitions are not student-constructed concepts, rather, they are instructor-provided vocabulary.
Anomalous Results
Unanticipated laboratory results are common in school science labs; in introductory physics, however, troubleshooting is often straightforward. Our attention in physics is on constraining the system as much as possible to focus on a single, pairwise interaction. Because of this, inconsistent results are generally a result of student error and not due to variability inherent to all experimental work. Furthermore, experiments involving rolling carts, colliding objects, dropped balls, and attracting magnets can easily be replicated and consensus quickly reached. As a result, students in the PET course frequently pursue investigations in small groups with a chance to share results at the end of the lab and with infrequent problems in achieving anticipated results.
In biology, however, the multitude of interactions underlying the processes we investigate (photosynthesis, decomposition, growth, cellular respiration) are so complex, and involve live organisms responding with inherent variation, that unanticipated results are frequently due to variability that cannot be controlled in our lab setting and are not due to errors in implementing procedures. We quickly found that small-group work in teams of four led to inconsistent results and required groups to compile and discuss data as a whole class before proceeding. Instructors must actively facilitate these discussions to call attention to how variability may play a role, what kinds of trends we are seeing, whether or not those trends make sense, and whether we can account for the variation.
Incorporating Scientists’ Ideas
In contrast to typical textbooks for introductory science, and consistent with best practices for science education (Bransford et al., 1999), both PET and LSET ask students to construct their own ideas through investigations and discussions with peers. To present canonical scientific descriptions of these ideas, students compare their ideas with scientists’ ideas. In PET, this happens at the end of each chapter, where students generally find that the ideas they have constructed are congruous with scientific principles. In LSET, however, we found that there were details that could not easily be constructed by students through laboratory investigations; certain background information needed to be presented earlier to enable students to move forward though the curriculum. For example, in chapter 2 ATP is introduced without a laboratory investigation, which might allow students to discover for themselves its role in biological systems, and it is also introduced before the end of the chapter. Students are asked to make sense of the idea and explore why this intermediate molecule is necessary to link cellular respiration and cell work. It was necessary to “tell” about this idea in the middle of the chapter, because the concept was necessary for building understanding of upcoming concepts, yet it was difficult to allow students to “discover” it for themselves.
Again, we can attribute this difference to the nature of the two courses; in a course attending to pairwise interactions, there are few hidden mechanisms to introduce. Within introductory biology, some facility with the mechanisms underlying the key processes is useful, but not always feasible or practical to introduce through student-centered investigations, especially when the mechanisms are molecular in nature and are thus difficult to investigate directly in an introductory course. In the case of ATP, we relied on an analogy (one of miniature rechargeable batteries) to introduce the concept through a Scientists’ Ideas section. Introducing those ideas early on in the chapter proved useful.
Assessing LSET
PET has proven itself as a curriculum that promotes strong understanding of core ideas surrounding energy in physics (Goldberg et al., 2010). A key question in evaluating the LSET curriculum centers around whether this curriculum—modeled on PET but with biological content and with changes in pedagogy necessitated by the shift to a processes view of science—would have similar success.
By way of example, the following assessment question exemplifies the big picture understanding that introductory biology courses seek:
Grandma Johnson had very sentimental feelings toward Johnson Canyon, Utah, where she and her late husband had honeymooned long ago. Because of these feelings, when she died she requested to be buried under a creosote bush in the canyon. Describe below the path of a carbon atom from Grandma Johnson's remains, to inside the leg muscle of a coyote. NOTE: The coyote does not dig up and consume any part of Grandma Johnson's remains. (Ebert-May et al., 2003, p. 1224)
This question requires students to integrate and apply their ideas about many linked biological processes—decomposition, photosynthesis, food webs, and carbon cycling. We know that students often have misconceptions about these topics. For instance, many students initially believe that decomposition is a natural physical change that dead matter undergoes, in which wind and water break organic matter down into smaller pieces, as though it went through a paper shredder. Our goals were for students to understand that decomposers actively and chemically change the dead material into new, small molecules through cellular respiration (Smith and Anderson, 1986). Similarly, many students believe that photosynthesis provides energy for plants to take in organic molecules such as glucose and amino acids through their roots, rather than serving as the process by which such organic molecules may be constructed from carbon dioxide, water, and minerals in the soil (Taylor et al., 2012).
Complete and correct understanding of carbon cycling as measured by the Grandma Johnson question, or others like it, would be difficult to attain by attending to pairwise interactions or even by attending to the complex molecular interactions that occur within mitochondria and chloroplasts (such as the set of chemical reactions in glycolysis). Yet, by attending to linked processes of photosynthesis, cellular respiration, and decomposition, big picture understanding appropriate to an introductory biology course can emerge.
METHODS
To determine whether students who use the newly developed LSET curriculum gain the desired big picture understanding of central life sciences processes, we assessed our students’ responses on the Grandma Johnson question (Ebert-May et al., 2003). Four groups of students were asked the Grandma Johnson question on a final exam.
High school: Students in grades 9–12 who either took a year-long biology (n = 26) or advanced placement (AP) environmental science (n = 20) course in public high schools in Bellevue, Washington. These high school courses could be characterized as typical high school science courses that adopt a fairly traditional, teacher-centered, pedagogical approach with a combination of whole-class lecture and small-group labs. The biology course was required of all 9th grade students for graduation. All students enrolled in the AP environmental science course were 11th or 12th graders. | |||||
College: Community college students from Everett Community College, Everett, Washington, who took either a quarter-long introductory biology for nonmajors (n = 23) or an introductory environmental science (n = 11). These college courses could be characterized as typical college, nonmajors science courses, although the environmental science class would typically have more of an emphasis on matter and energy transfer than would the general biology class. The two groups of students were very similar in terms of their background (all were nonscience majors.) We pooled their data because the number of students enrolled in the environmental science course was low, the students were all from the same institution, both courses were for nonmajors, and their data were very similar. | |||||
Quarter LSET 1.0: Community college students from Everett Community College, Everett, Washington, who took the original quarter-long version of the LSET curriculum (n = 26). Students in this class are not science majors and have minimal science background. Approximately 33–50% of the students in the class plan to become elementary school teachers. These data were collected early in the development of the curriculum, prior to adding an additional 5 wk of curriculum to address cell biology and genetics. However, the bulk of the questions, discussions, and activities relevant to cellular respiration, photosynthesis, decomposition, and energy and matter cycling were completed by this time. | |||||
Semester LSET 2.0: College students from CSU, Chico who took the semester-long version of the LSET curriculum (n = 45). Students in this class are not science majors and typically have minimal science background. Approximately 95% of the students intend to become K–8 teachers. These data were collected late in the development of the curriculum and are representative of the revised version being disseminated currently. | |||||
The results were scored according to the coding scheme shown in Figure 6A, which was derived from a grounded coding of student responses. Each student's response was evaluated for the presence or absence of major target biological processes (A: decomposers consume Grandma Johnson's remains and perform cellular respiration, releasing carbon dioxide into the environment; B: a plant takes in carbon dioxide and makes glucose through photosynthesis; C: an herbivore consumes the plant; D: the coyote consumes another organism) and those of common misconceptions (W: the coyote consumes a plant; X: a plant absorbs matter from Grandma Johnson's remains through its roots; Y: worms or other detritivores consume Grandma Johnson's remains; Z: the coyote consumes Grandma Johnson's remains). A blank, vague, or nonsensical answer was coded N. If a pathway was omitted, it was not given a code as part of either a correct or incorrect answer. See Table 3 for example student responses and a brief description of how each was coded. The two of us responsible for coding the answers communicated closely on the coding scheme. Interrater reliability was established by both of us coding 46 of the same student responses and comparing codes. Interrater reliability was 93.5%, with a Cohen's kappa statistic of 0.73.
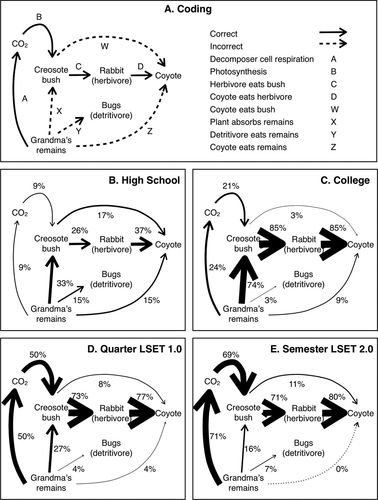
Figure 6. Qualitative Grandma Johnson assessment data. (A) Coding scheme for open-ended student responses. ABCD is the complete correct pathway. (B–D) Student responses for each of the four groups of students. The line width of the arrows is proportional to the percentage of students that mention a given process.
| Example student response | Score | Notes |
|---|---|---|
| Her body would have its energy absorbed into the ground and then that energy is moved from the ground into the coyote's leg because of recycled energy and recycled matter. | N | This vague and nonsensical answer confuses energy with matter. |
| Grandma Johnson decomposed into various nutrients which entered into the soil of the ecosystem. These nutrients were used by plants to grow. These plants were then eaten by a consumer, such as a rabbit. That rabbit was then consumed by the coyote. | XCD | This student thinks that decomposed matter enters plants through their soil. |
| The decomposer would eat her carbs and then the decomposer would gain carbs. Through cellular respiration by the decomposer carbon would be released into the environment. The coyote would then eat a decomposer which has carbs and now the coyote has it's own carbs. | ADY | This student knows that a decomposer can perform cell respiration, however, misses the role of plants. |
| Her matter is used by the plant (CO2 for example), which is eaten by the herbivores, which is eaten by the coyote. This matter is used to perform certain tasks, and in this case performing a muscle leg of a coyote. | BCD | This incomplete answer fails to account for how carbon dioxide was generated. Because a decomposer was not mentioned, that pathway (A) was not coded. |
| Grandma Johnson (now a dead organism) would store the carbon atom as a carbohydrate; when she is eaten by bacteria (a decomposer) the carbon atom will transfer to the bacteria as a carbohydrate. After cellular respiration the carbon atom will be released into the atmosphere as a CO2 molecule. This CO2 molecule will be used by a plant during photosynthesis and will be stored as a starch within the plant. The plant will then be eaten by a mouse who will store the carbon atom in the form as [sic] glycogen. When the mouse is eaten by a coyote the carbon atom will be transferred to a muscle cell in the coyote's leg. | ABCD | This example of correct understanding illustrates a complete and accurate account of the carbon cycling in this scenario. |
In addition to a qualitative assessment of these data, we conducted an independent samples Kruskal-Wallis test to determine the level of differences between the response distributions across the four courses (high school, college, quarter LSET 1.0, and semester LSET 2.0) and whether there were any differences between the percentage of students that identify a correct pathway (ABCD) versus the most common incorrect pathway (XCD), all other incorrect pathways, or nonanswers (N) in each group.
RESULTS
The analysis of the Grandma Johnson data showed clear differences between the responses of students who were and were not exposed to the LSET curriculum (see Figure 6, B–E).
High school students completing an introductory biology course or an AP environmental science course showed little understanding of carbon cycling. Twenty-two percent of the students provided vague, incoherent responses, such as: “I don't know” and “The carbon atom is broken down and Grandma Johnson eventually decomposes.” Only one student was able to identify the complete correct pathway.
College students in introductory biology and introductory environmental science were less uncertain (only 3% were scored N), but, on the whole, gave a surprising number of incorrect answers. Seventy-one percent of these students assumed that the plant absorbed carbon atoms from Grandma's remains through their roots.
Performance on this question improved dramatically with the LSET curriculum. Forty-six percent of students taking the early, quarter-long version of the course and 64% of students taking the revised, semester-long version of the course were able to identify the complete correct pathway.
An independent samples Kruskal-Wallis test showed that the distributions of student responses (correct, incorrect [XCD], incorrect [all others], or blank/vague) across the four courses were not statistically similar (p < 0.01 level). In other words, the distribution of responses changed significantly as students matured (between high school and college) and between students who took different versions of the LSET curriculum (between college, quarter LSET 1.0, and semester LSET 2.0). These data are represented in Figure 7.
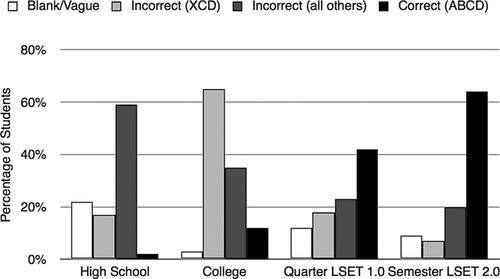
Figure 7. Quantitative Grandma Johnson assessment data. Students exposed to the LSET curriculum were more likely to describe the correct pathway than high school or college students with more traditional biology or environmental science curricula.
DISCUSSION
As we strove to create an exemplary life sciences curriculum modeled after PET, we anticipated that the format would adapt well to biological content; the PET curriculum is based on learning principles that are largely independent of content, and we therefore expected that many aspects of the curricular design would transport easily and well. For example, both curricula place great emphasis on eliciting, acknowledging, and explicitly addressing students’ commonly held initial ideas. Similarly, the daily structure of PET lessons with their emphasis on cooperative learning and hands-on experimentation flowed readily into the life sciences context.
However, there were also considerable challenges to overcome—challenges that would likely impact any effort to adapt proven curricula from physics education to fit the needs of biology at the introductory level. Primary among these considerations is a difference in whether the curriculum emphasizes pairwise interactions or linked processes involving many interactions. Whereas introductory physical science presents itself as offering explanations and mechanisms at the level of individual interactions between objects and molecules, introductory biology considers systems such as living organisms and ecosystems that are far more complex. Introductory biology does not attempt to offer explanations at the level of pairwise interactions—there are simply too many to consider for a nonscience major. Rather, understanding processes at the big picture level of organisms and ecosystems becomes a far more relevant and important goal.
The consequences of targeting ideas at the process level is that many features of well-developed physics curricula such as PET required significant reworking to find success in a life sciences context. Representations of energy transfers cannot represent pairwise interactions—rather, biology must focus on processes. Definitions cannot be presented as a simple, straightforward given—constructing definitions becomes part of the scientific inquiry, embedded within an understanding of significant biological processes. Anomalous experimental results cannot simply be attributed to experimental error—live organisms, with innumerable interactions underlying the processes we investigate, do not always behave in anticipated ways, and isolating particular interactions is neither feasible nor does it address core introductory biology topics. And scientists’ ideas cannot wait until the end of a chapter—some biological ideas need introduction and cannot be constructed from first principles in the introductory course.
Successfully addressing these challenges affords superior student outcomes in that students may then fully explore and come to understand central biology concepts from a big picture perspective. After completing our LSET curriculum, our students can integrate and apply concepts from across life sciences topic areas (such as photosynthesis, decomposition, and ecology) better than high school or college students who completed more traditional biology and environmental science curricula, as we found with the results of the Grandma Johnson question. Students who had taken LSET had a better understanding of the core concepts of the flow of matter and energy in living systems and could relate them to the fundamental principles of the conservation of energy and matter.
This is partly due to the pedagogy of the course. As is advocated in best practices of biology education (and science education in general), students are afforded time to wrestle with ideas and to construct understanding. Prior knowledge is elicited and explicitly addressed. Students are prompted to record how their thinking has changed. However, we think the success of our students is also due to how our course is thematically and pedagogically linked with PET. Linking PET and LSET allows students to better use principle-based reasoning, especially in terms of the flow of energy and matter. PET explicitly covers the law of conservation of energy in the use of energy diagrams. By adapting energy diagrams into our curriculum, and by adding matter diagrams, we enabled students’ exploration of the connections between these laws of physics and the processes of biology.
The curriculum also allows students to learn in a manner that addresses some of the reasons elementary teachers feel unprepared to teach science. Rather than presenting biology as a collection of facts to be memorized, LSET presents it as a way to understand the living world. Students come to understand that biology is about asking and answering questions. This process is modeled for them throughout the course. Additionally, the Learning about Learning activities explicitly link what they will experience in the curriculum in their future classrooms.
Physics has a long tradition of research-based curricula at the undergraduate level, with methods that may be leveraged for use in developing curricula for undergraduate biology. However, leveraging these approaches requires more than importing biology content into formats established in physics. Attention to the ways in which pedagogical innovations support particular disciplinary norms and how those norms differ between disciplines is critical for adapting innovations into new fields.
ACKNOWLEDGMENTS
We are grateful to the many students who have been in our classes and have given us helpful feedback on this curriculum and to our colleagues who were part of the development team. This work was funded through NSF grants 0315060 and 0942391. The manuscript was greatly improved by thoughtful comments from two anonymous reviewers.
FOOTNOTES
†These authors contributed equally to this work.
Statement of disclosure: All of the authors of this paper were involved in developing the curriculum that is the focus of this study. The curriculum is not yet commercially available but can be obtained by contacting the corresponding author. No promotion of this curriculum to the exclusion of other similar products should be construed.
Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Science Foundation.



