“Big Ideas” of Introductory Chemistry and Biology Courses and the Connections between Them
Abstract
Introductory courses are often designed to cover a range of topics with the intent to offer students exposure to the given discipline as preparation to further their study in the same or related disciplines. Unfortunately, students in these courses are often presented with an overwhelming amount of information that may not support their formation of a usable coherent network of knowledge. In this study we conducted a mixed-method sequential exploratory study with students co-enrolled in General Chemistry II and Introductory Biology I to better understand what students perceived to be the “take-home” messages of these courses (i.e., core ideas) and the connections between these courses. We found that students identified a range of ideas from both courses; further analysis of students’ explanations and reasoning revealed that, when students talked about their chemistry ideas, they were more likely to talk about them as having predictive and explanatory power in comparison with reasons provided for their biology big ideas. Furthermore, students identified a number of overlapping ideas between their chemistry and biology courses, such as interactions, reactions, and structures, which have the potential to be used as a starting place to support students building a more coherent network of knowledge.
INTRODUCTION
The goal of any science educator is to prepare students with sufficient meaningful and robust knowledge to support their growth as science learners, consumers, and even scientists (Bell, 2001; National Research Council [NRC], 2012a; Krajcik and Delen, 2017). While it would not be expected that students become disciplinary experts after one or two semesters of introductory courses, ideally, they should start gaining a foundation that supports their development of scientific knowledge. These early science courses are often designed to cover a wide range of topics with the intent of offering beginning students exposure to the given discipline as either preparation to further their study in said discipline or related disciplines (Alberts, 2012). The Discipline-Based Education Research report highlighted, based on years of evidence, that introductory science curricula structured to consider a discipline’s breadth instead of depth does not lead to the development of a coherent framework on which students can build their knowledge (NRC, 2012b). Furthermore, the overwhelming amount of information covered in these courses leaves little room to support students’ formation of a usable coherent network of knowledge in which they can build connections between topics, much less across disciplinary concepts. As a result, students tend to leave their introductory courses with limited usable and transferable knowledge; thus, these courses are failing to prepare them for advanced courses or future careers or to be scientifically literate consumers.
Many reform efforts have focused on how to teach (pedagogy; Gafney and Varma-Nelson, 2008; Moog and Spencer, 2008; Freeman et al., 2014); however, in recent years, there has been an increase in the number of initiatives that focus on what students are learning in addition to how they are being taught. These initiatives seem to revolve around the identification of “core” (big/central/foundational) ideas within disciplines. A Framework for K–12 Science Education (referred to hereafter as the Framework) is one of the most ambitious initiatives to date that revolves around core ideas (NRC, 2012a). The Framework report synthesized the research on how people learn and highlighted what students should know (core ideas) and should be able to do with their scientific knowledge (scientific practices) and the concepts that should be used as lenses, tools, and bridges to support the understanding of disciplinary knowledge (crosscutting concepts; Cooper, 2020). Similarly, other initiatives focus on defining central ideas for multiple disciplines at various levels. These initiatives include the Advanced Placement for high school chemistry (Rushton, 2014; College Board, 2020), the American Chemical Society for chemistry courses at the college level (Holme and Murphy, 2012; Holme et al., 2015, 2018; Holme, 2020; Raker et al., 2013; Marek et al., 2018), the Vision and Change report for life sciences (American Association for the Advancement of Science [AAAS], 2011), and the American Society for Biochemistry and Molecular Biology list of foundational concepts (ASBMB, 2015).
The study presented herein is part of a larger project with the aim to investigate students’ perceptions and understanding of core ideas in their chemistry and biology courses. Within this mixed-method study, we first interviewed 28 students who were co-enrolled in introductory chemistry and biology courses regarding their perspectives on the “take-home” messages or big ideas from these two courses. We also explored how students perceived the connections among the big ideas. Next, to understand how representative the interview findings were among a larger group of students, we developed and administered a survey to explore students’ perceptions of the chemistry and biology big ideas.
Core Ideas at the Center of Introductory Courses
The Framework defines core ideas as central to the discipline, providing underlying support for a wide range of concepts across the discipline, and most importantly, as having explanatory and predictive power (NRC, 2012a). Core ideas provide students with the organizational structure that supports the acquisition of new knowledge to construct more expert-like knowledge structures. Expert knowledge is not simply a list of isolated facts or propositions that are relevant to their domains; instead, such knowledge is organized around core concepts or big ideas that allow experts to connect and use knowledge in new situations (NRC, 2000). Thus, centering introductory courses around core ideas rather than separated topics provides students with the opportunity to build a network of ideas that are connected and contextualized.
With this in mind, the Framework was adapted at the institution of interest in an effort to transform the introductory chemistry, biology, and physics courses. Faculty at the institution of interest identified the core ideas of gateway courses as part of transformation efforts (Table 1) that focus on what students are expected to learn in these courses at the introductory level and what they are expected to know going into upper-level courses (Laverty et al., 2016; see course descriptions in the Setting and Participants section).
| Chemistry core ideas | Biology core ideas | Physics core ideas |
|---|---|---|
|
|
|
While there is a large body of research focused on identifying students’ difficulties within a discipline and the impact of pedagogical practices on their performance; less is known about what students are learning, what they consider to be important, how they use that knowledge in new situations within the course, and how they relate and connect their knowledge to other disciplines. As part of a larger project with the aim of identifying and understanding connections and potential barriers between introductory chemistry and biology courses (Kohn et al., 2018a,b), the work presented in this article explores what content students remember from their courses, what they perceive to be take-home messages of the courses, and how they think their introductory chemistry and biology courses are related. This work investigates students’ perceptions of the big ideas for two introductory courses that have undergone or were in the process of a curricular transformation with a focus on core ideas. Therefore, the data presented here should be considered in the context of these transformed courses (see description of courses in Setting and Participants).
Identifying what students learned from their chemistry and biology courses and what they perceived to be important is essential to understand the extent to which students are able to recognize the central ideas of these courses and how the current states of these courses support students’ knowledge framework around core ideas and to identify opportunities that curriculum developers and instructors could use to help students solidify their understanding of core ideas within the courses and make connections between them.
Research Questions
The research presented here is guided by two research questions:
What do students consider to be the big ideas of their introductory chemistry and biology courses along with the reasoning behind their perceptions?
What ideas do students identify as overlapping in both their introductory chemistry and biology courses?
METHODS
These research questions were answered using a mixed-method sequential exploratory design (Towns, 2008) consisting of qualitative semistructured interviews followed by the development and administration of a survey to explore the distribution of such findings across a larger group of students.
Setting and Participants
The data collection for this study took place at a large, public research-intensive institution in the U.S. Midwest. The participants included were co-enrolled or had previously taken both semesters of general chemistry and one semester of introductory biology at the time of the data collection. At this institution the two-semester general chemistry (GC1 and GC2) course sequence enrolls an average of 4000 students every year, with about 350–450 students per lecture section. During the time of this study the institution of interest had implemented a transformed general chemistry curriculum known as Chemistry, Life, the Universe and Everything (CLUE; Cooper and Klymkowsky, 2013). CLUE was designed based on evidence of how people learn (NRC, 2000) and focuses on what we want students to know upon exiting the course sequence (chemistry core ideas: electrostatic and bonding interactions, atomic/molecular structure and properties, energy, and change and stability in chemical systems; Laverty et al., 2016) and what students should do with their knowledge (scientific practices such as developing and using models and constructing explanations; NRC, 2012a). Students in this course use an open education resource textbook written by the curriculum developers and complete homework using beSocratic, which is an online assessment platform where students can submit written, drawn, and graphical responses (Cooper et al., 2014). In addition to the lecture time, students attended a 1 hour required recitation section consisting of about 30 students in each section where they worked in small groups to complete worksheets to support their understanding of ideas presented during lecture.
The introductory biology course at the same institution (B1) mostly focuses on cell and molecular biology and enrolls about 2300 students per year, with 150 to 250 students per lecture section. Similar to the GC1/GC2 courses at this institution, B1 was undergoing a transformation with their curriculum inspired by the Vision and Change report (AAAS, 2011). As part of this transformation, B1 instructors were beginning to place emphasis on seven biology core ideas (chemical and physical basis of life, matter and energy, cellular basis of life, system, structure and function, information flow, exchange, and storage, evolution), which were identified by the faculty at the institution (Laverty et al., 2016). B1 students were presented with these seven core ideas at the beginning of the semester and were reminded of the applicable core ideas within context for each unit. This course used a commercially available textbook and associated online homework system (Mason et al., 2015); the lectures incorporated in-class activities and students participated in five modeling activities throughout the semester. In these modeling activities, students were asked to construct representations of a system and then predict and explain the biological processes involved.
Data Collection
The details of the interview and survey portions of the study are presented here. Both portions were approved by the institutional review board (IRB Institution, Michigan State University) before data collection, and all students were notified of their rights as participants of the study and were provided information about the project.
Interview Details
For the interviews, all participants attended the same preselected lecture section of B1 to compare students with similar experiences at the end of Spring 2014 and Spring 2015 semesters. That is, these students needed to be co-enrolled in B1 and GC2 at the time of the interview. Interviewed students were offered a small amount of extra credit as compensation for their time, and those who volunteered to be interviewed, but did not meet these criteria, participated in a different activity for the extra credit. A total of 28 students (15 self-identified as females and 13 as males) whose course grades ranged from 1.5 to 4.0, with a total of 16 students who earned a 3.0 or above in GC1, GC2, and B1 were included (see Supplemental Material S.1 for student demographics). During the semistructured interviews students were given a Livescribe smartpen to collect detailed recordings of their drawings, writing, and audio in real time (Linenberger and Bretz, 2012) to capture an accurate perception of the interviewees. While the interviews were expected to last about an hour in length, students were willing to stay longer than anticipated to complete the interviews, as they were excited to talk about their courses; therefore, the interviews typically lasted between 70 and 150 minutes, depending on the amount of information provided.
Interview protocol: The interview protocol was designed to identify opportunities for connections between the GC1, GC2, and B1 courses as well as potential barriers in developing those connections as part of a larger study. It should be noted that students were asked to think of their two chemistry courses as a single course (G1/G2 course) to streamline student discussion and the analysis process. The interview guide for this larger project had four phases (Figure 1) and has been previously published (Kohn et al., 2018b). The first phase asked students to talk about their college experiences thus far and their future plans and to comment on any science courses taken during high school. Phase 2 asked students to reflect on their GC1/GC2 experiences by brainstorming a list of “things” they learned during the courses and then asked the participants to identify and explain the big ideas or take-home messages of their chemistry course. In the interview protocol, it was decided to present students with general language of “big ideas” and take-home messages, because the language of “core ideas” would be less familiar to them. Therefore, no distinction was made between the terms “big ideas” and “core ideas” during the data-collection and analysis process. For phase 2, students repeated the same set of tasks (i.e., listing things learned, identifying and providing reasoning for big ideas) for their B1 course. In phase 3, students were asked to compare their courses by describing and explaining any connections they perceived between the courses. Finally, phase 4 asked students to generate a list of “themes” that they believed span chemistry and biology and to discuss how energy and the relationships between structure, properties, and function were presented in both their GC1/GC2 and B1 courses. Findings from phases 2 and 3 of the interviews (students’ reflections on courses and connections between courses) will be presented here, as the findings on students’ ideas about the crosscutting concepts in phase 4 has been previously reported and discussed (Kohn et al., 2018a,b).
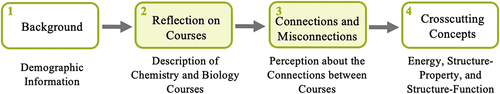
FIGURE 1. Interview protocol phases. Findings from phase 4 were reported in Kohn et al. (2018 a,b).
Survey Details
Phases 2 and 3 from the interview guide (Figure 1) were modified to develop a survey (Figure 2) that was administered via Qualtrics at the end of the Spring 2016 semester to students enrolled in GC2. Out of the 815 students registered in the GC2 course, 109 students completed all four questions of interest on the perceived usefulness of the topics from their chemistry courses (GC1/GC2) and biology course (B1). Responses from these 109 students (70 self-identified as female and 39 as males) who had a course grade average of 3.18 (range from 0.0 to 4.0) were used for the data analysis. The students included in our analysis were representative of the students registered in the course (see Supplemental Material S.1 for student demographics).
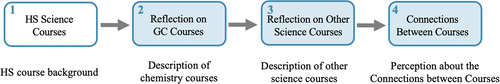
FIGURE 2. Structure of the survey administered via Qualtrics during the quantitative portion of the study.
Surveystructure: In part 1 of the survey, students were asked to reflect on their science courses taken during high school (Figure 2). Part 2 asked students to describe their experiences in GC1/GC2 along with their perceptions on what they learned in these courses. The third part of this survey asked students to indicate what other science courses they had taken (i.e., biology or physics) and to describe their experiences in these courses and what they learned in them. Finally, part 4 asked students to draw connections between the other science courses they identified as having taken and GC1/GC2. While physics was part of the survey with a larger goal to explore understanding among various science courses, the study presented in this paper only included the students’ chemistry and biology courses, as this allowed us to compare our interview findings with the survey data. For example, the findings presented here focus on how students were asked to describe any ideas or topics covered in the General Chemistry 1 (GC1) course that were useful for thinking about Cell and Molecular Biology (B1) and vice versa. The findings from parts 2 through 4 of the survey (Figure 2) will be reported in this paper. It is important to note that students were asked in part 4 to reflect on their perceptions regarding the topics learned in their individual general chemistry courses (GC1 or GC2) that they found helpful to think about B1, or vice versa; thus, a total of four questions exploring the perceived connections between these courses were posed to the students (i.e., GC1 → B1, GC2 → B1, B1 → GC1, and B1 → GC2). A list of relevant questions for the survey can be found in the Survey Summary (Supplemental Material S.2).
Data Analysis
The details of analytical methods for both the interview and survey data are first described before presenting the common coding scheme developed for both the interview and survey data. The coding scheme (presented in Figure 3) was developed from the interview data and then applied to the survey data. Detailed audit trails (Bretz, 2008) of any changes and methods used to analyze the interview data or survey data were kept.
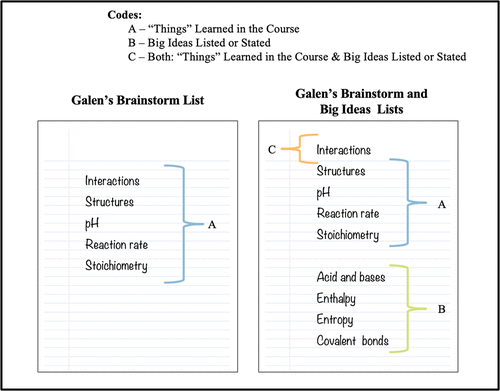
FIGURE 3. Example of how individual students’ lists of topics and ideas written or stated were coded.
Interview Analysis
All interviews were transcribed verbatim by a professional transcription service, then reviewed and edited for both accuracy and completeness by author K.P.K. Both the written responses and interview transcripts were used to analyze the student brainstorming process and big ideas identified by the students for the analysis process of the interviews. Students were assigned pseudonyms to protect their identities, and Excel was used to manage and code the data generated from the interviews. Authors Z.D.R.A, L.S.C., and B.P. held weekly meetings to discuss and revise the data analysis protocol and codebook to ensure the accuracy of the interview data analysis. The descriptions generated for the codebooks used students’ language to accurately capture and present what students described and talked about during the interviews (see Codebooks in Supplemental Material S.3 and S.4). Unlike the survey questions, interview questions asked students to explain why they thought the ideas they listed were big ideas. It is important to note that students were only asked to provide a general reasoning for “why” they listed their big ideas and not specifically for each idea listed. Constant comparative analysis (Strauss and Corbin, 1998) was used to identify why students thought the listed ideas were the big ideas of their GC1/GC2 and B1 courses. That is, student explanations and descriptions were compared with one another to identify any similarities and differences among them.
Survey Analysis
The analysis of the survey data began by identifying all students who completed the four questions regarding the connections perceived between their GC and B1 courses. This data cleaning and managing process took place in Excel, where the data was sorted and students who did not answer the questions of interest were removed for data analysis purposes. Discussions and revisions to the data analysis protocol and codebook were conducted by authors Z.D.R.A. and A.E.G. through biweekly meetings to ensure the accuracy and consistency of the survey data analysis (see Codebooks in Supplemental Material S.5 and S.6). It should be noted that the questions exploring what students perceived from GC1 and GC2 were asked separately as part of the survey; however, the data from these questions were combined to be consistent with the analysis of the interviews. Similar to this process, students’ responses to the connections perceived between GC1 and B1 and GC2 and B1 were combined to explore the connections perceived between their overall chemistry and biology courses, rather than individual semesters.
Common Coding Scheme for Interview and Survey Data
Although students were asked to create two separate lists—one for the list of things learned in the course and one for their big ideas or take-home messages—students would occasionally repeat topics or ideas when asked to address the latter. The coding process was simplified to avoid double counting by first identifying all the things and big ideas each student listed or mentioned during the interview and then classifying them as: A) Things learned in the course (brainstorming list), B) big ideas listed or stated, or C) both. Here, we use an example from Galen’s interview (Figure 3) to explain the coding scheme used for the interview and survey data.
When Galen was asked to provide a list of the things he learned during his GC1/GC2 course, he listed “interactions, structures, pH, reaction rates, and stoichiometry” (Figure 3, Galen’s brainstorming list). After providing a description of what he listed, Galen was asked to provide a list of what he thought were the big ideas or take-home messages from his GC1/GC2 course. As depicted in Figure 3, Galen continued with his list of big ideas, which included “acid and bases, enthalpy, entropy, and covalent bonds,” but he also stated that “interactions” were a big idea in his course without rewriting it as part of his big ideas list. Therefore, as part of our analysis, only structures, pH, reaction rate, and stoichiometry were counted and labeled as things learned in the course (A), while acid and bases, enthalpy, entropy, and covalent bonds were counted and labeled as big ideas (B), and interactions was counted and labeled as both (C). The same coding process was used for the students’ B1 course lists.
For both the interviews and surveys, a few students did not provide specific things or big ideas learned in their course, instead saying things like: “I learned the basics of chemistry” (Survey Student 271).
In addition, some of students expressed feeling unsure of what they learned or what the big ideas of the GC1/GC2 course were. Thus, all of these students were coded as “No specific idea provided,” because they did not talk about any specific content. It should also be noted that some students who were coded as having zero big ideas (B), identified ideas as both things learned and big ideas; therefore, they were coded as both (C) instead.
RESULTS AND DISCUSSION
The purpose of this study was to investigate students’ perceptions of what they learned from their individual introductory chemistry and biology courses. The following subsections present students’ perception of the big ideas from their GC1/GC2 and B1 courses, their reasoning, and their views of how chemistry and biology ideas overlap. For consistency, for the remainder of the article, when we refer to “things learned,” this could include any topic, concept, idea, and even a skill a student might have mentioned. “Big ideas” will be used in reference to what students stated were take-home messages. The term “overlapping ideas” will be used to refer to any themes, topics, concepts, ideas, and skills a student might have perceived as a connection between their courses. Finally, “core ideas” will be used to refer to the list of ideas identified by faculty as being central to their disciplines.
Research Question 1: What Do Students Consider to Be the Big Ideas of Their Introductory Chemistry and Biology Courses along with the Reasoning behind Their Perceptions?
Both the interview and survey data revealed a large range of unique things that students identified as having learned in their GC1/GC2 (n = 57 for interviews and n = 56 for surveys) and B1 courses (n = 53 for interviews and n = 40 for surveys). Fewer ideas were presented when the students discussed their big ideas for these courses. Table 2 highlights the top five big ideas identified by students from their GC1/GC2 and B1 courses in the interview data, which are similar to the lists created from student responses to the survey (see more details in Supplemental Material S.7–S.9). Further, within the interviews, students mentioned between one or two big ideas on average for both courses. In many instances, the big ideas listed encompassed exact phrasing of the core ideas listed by the faculty in Table 1. For example, in chemistry, interactions (47%, n = 13) and types of bonds (22%, n = 6) are part of the core idea of electrostatic and bonding interactions and 15% of the students listed energy (n = 5; see Supplemental Material S.9A). This finding was also observed for students’ biology courses through structure and function which was captured verbatim by the interviewed students (36%, n = 10) as a big idea. While it was promising that students were able to state some of the core ideas identified by the faculty, there were other big ideas listed by students that could be connected to a core idea from the course depending on how students were thinking about it. For example, in chemistry, reactions (43%, n = 12), structures (25%, n = 7), acids and bases (11%, n = 3), and reaction rate (4%, n = 1) could all be part of the larger core idea of atomic/molecular structure and properties or they could be isolated things in the students’ minds. This is also true for students who mentioned reaction equilibrium (36%, n = 10), which could be part the core idea of change and stability in chemical systems. In biology, mutation (32%, n = 9; see Supplemental Material S.9B) could either be considered within the core idea of structure and function, if students were thinking about how a change in the structure (i.e., mutation) could lead to a different function, or it could be within the core idea of evolution, if students were thinking about how evolution results due to changes in structure (i.e., mutations).
| Top 5 big ideas | |
|---|---|
| Big ideas listed during the interview | Chemistry: no. of students (%) |
| Interactions | 13 (47) |
| Reactions | 12 (43) |
| Structures | 7 (25) |
| Types of bonds | 6 (22) |
| Periodic trends | 5 (18) |
| Big ideas listed during the interview | Biology: no. of students (%) |
| Structure–function relationship | 10 (36) |
| Cell respiration | 8 (29) |
| Cell organelles | 6 (21) |
| DNA | 6 (21) |
| DNA replication | 6 (21) |
Regardless of the course, whether the students’ listed big ideas can truly be considered core ideas is highly dependent on how they thought about and discussed these within the interview. That is, core ideas should be explanatory in power, generative of new ideas, and central to the discipline (NRC, 2012a). However, for the survey, students were not asked to provide any reasoning for the big ideas considered to be important for their courses. Thus, analysis of student reasoning only pertains to the data collected during the interviews. When asked to provide a reason for their big ideas, students were also asked to provide a general reason why they thought their lists represented the big ideas from the GC1/GC2 course, followed by the B1 course. Although students included a variety of things learned as part of their lists, they were not asked to provide a reason for each individual big idea listed. It should also be noted that students were not restricted to providing only one reason. In fact, on average, each student provided one to two reasons for each course, with a range from zero to four.
The array of reasoning provided by students was coded and counted as shown in Figure 4 for responses with 25% or more of the students. It was not surprising that students identified big ideas that were re-occurring within a course (43%, n = 12 for GC1/GC2; and 36%, n = 10 for B1), important or central for other concepts (11%, n = 3 for GC1/GC2; and 25%, n = 7 for B1), or that they had spent more time on over other ideas within the course (32%, n = 9 for GC1/GC2; and 18%, n = 5 for B1), as would be expected if a big idea was truly a core idea for a course. That is, time would be spent on that core idea by building connections on how other things learned would be related to that core idea. Students also stated that the instructor often identified the big ideas for the course (25%, n = 7, Figure 4); however, this was only for the B1 course, as the instructor explicitly identified the core ideas of the course at the beginning and throughout the semester. Upon prompting for further explanation, not all students knew why the big ideas they listed from their B1 course were important and instead only recalled that these big ideas were important. On the other hand, it was encouraging to see that some students talked about big ideas in a sophisticated manner, as having explanatory and/or predictive power (43%, n = 12 for GC1/GC2; and 7%, n = 2 for B1). These students stated that the big ideas listed had the potential to explain a wide range of phenomena within the courses that they would not have been able to explain otherwise. It was interesting that this reasoning was mentioned mainly for students in the GC1/GC2 course compared with the B1 course (Figure 4). We might surmise that the observed results are a consequence of the nature of their transformed chemistry course, because the goal of the course is to support the development of an understanding of core chemistry ideas so that students can understand and explain how and why chemical phenomena occur. In the following subsections, examples of students’ descriptions and reasoning for their big ideas listed will be discussed to better illustrate how students viewed their big ideas. See Supplemental Material S.10 and S.11 for a full list and counts of the reasoning provided by students from the interview data.
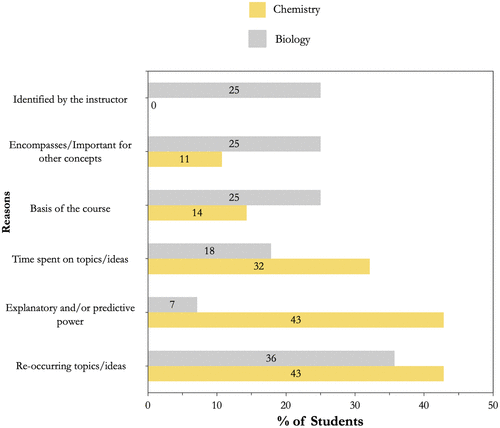
FIGURE 4. Reasons provided by 25% or more of the interviewed students explaining their rationale for listing/stating their big ideas.
Chemistry Example.
In a cross analysis of students’ big ideas and reasoning, it was found that some students provided laundry lists of big ideas for which they provided fragmented and surface-level descriptions, while others described how their big ideas could help them explain phenomena within the discipline. For example, the students’ responses for interactions in GC1/GC2 ranged from ambiguous textbook definitions to a list of examples of the types of intermolecular forces to a very small number of students indicating how interactions occur through electrostatic interactions. Let us compare Galen’s and Laura’s unprompted descriptions of interactions:
Galen: “It seems like the whole first semester was bonding and intermolecular forces, and that’s pretty much—I mean if you ask me to think of one big thing, that’s pretty much what I think of.”
Laura: “So, we spent a lot of time talking about intermolecular forces, which—the three examples we learned about were the London dispersion forces, dipole-dipole, and then hydrogen bonding… [We] learned about the different [potential energy] graphs and we learned how two atoms or molecules that might normally be neutral still interact and have the London dispersion forces present because of induced dipoles… Then I think dipole-dipole interaction is when there’s more of a permanent dipole in a molecule, so one part of it is positive or negative, and then that interacts with another molecule that also has the opposite dipole. That’ll be attracted. Or if it’s the same, it would be repulsed… Hydrogen bonding is an intermolecular force between an acidic hydrogen or one that’s attached to an electro-negative element or atom on one molecule, when that interacts with oxygen, nitrogen, or fluorine on the other molecule it is interacting with.”
Unlike Galen, who only identified interactions as being important for the course, Laura provided fine-grained details on how she believed each type of intermolecular force (IMF) occurs and the differences between them without being prompted to explain more in depth. Although these students provided very different descriptions of interactions, it was found that they both had similar reasons for believing that interactions represented a big idea. Galen stated that he believed interactions to be a big idea because of the time spent talking about them during the course, “Just because I feel that we talked about it the whole first semester.” As for Laura, she believed interactions to be the big idea of the course because of their reoccurrence, “I feel like that keeps coming back.” Students like Galen and Laura seem to recognize the importance of interactions in chemistry due to the frequency and the emphasis placed on the given idea. These speak to the nature of the chemistry course, in which electrostatic interactions are a core idea of the curriculum and are used to explain a range of phenomena (e.g., solutions and reactions). Students recognized that, in the course, they learned about interactions over time rather than this being an idea that was brought up once and never mentioned again.
Although it is noteworthy that students thought of interactions as a big idea because of the time dedicated to the concepts, one noticeable finding was the students who specifically talked about interactions as having explanatory and/or predictive power. A total of six out of the 13 students who considered interactions to be a big idea talked about how they could use electrostatic attractions to explain how [occurs at the molecular level], why [occurs at the molecular level], and “guess” what occurs at the molecular level when describing or analyzing a chemical phenomenon. Consider Karl, whose reasoning focused on the electrostatic nature of the different interactions.
Karl: “Like, if I didn’t know about these interactions, it would be a lot harder for me to understand why atoms are getting rearranged in the ways they are… I could just straight up memorize—sure this atom is going to switch off with this atom. The beryllium’s going to fly off and the sodium’s going to jump on there and something like that. But if I can think about what properties do these atoms have like where they sit on the periodic table or what type of electronegativity or effective nuclear charge they have and things like that, you can predict stuff a little bit better in what your course is. I should think it’s your general goal in the end.”
Students like Karl talked about the importance of understanding big ideas like interactions and how it allowed them to develop a more robust understanding of the relationship between atoms/molecules and their “behavior” to be able to predict both physical and chemical properties. Similar to Karl, other students specifically mentioned that, without “really understanding” interactions, they were just memorizing properties and how different phenomena occur.
The range in how students discussed interactions was also observed for the core idea of structure–property relationships. For example, many GC1/GC2 students mentioned structures, the majority referring specifically to Lewis structures, as being central to what they learned in the chemistry course. While Lewis structures by themselves are considered a “skill” that students need to master, students like Ruth thought that the ability to draw Lewis structures was a very important skill to develop as part of her chemistry course:
Ruth: “I put Lewis structures on there because I think the whole course also revolves around those, because you have to draw them to figure out most things that we learned.”
Ideally students would move beyond this notion of a Lewis structure being a skill and use the structure to predict and explain chemical and physical properties (the core idea of atomic/molecular structure and properties), like Shelly, a student who provided a description of Lewis structures as being more than merely a structure.
Shelly: “Because I mean we kind of just like draw the Lewis structure as the structure but that’s not the actual kind of structure … if it has the valence electrons like that makes it more reactive kind of thing… I seriously can only think of the whole structure thing because it boils down to everything really if you think about it. Like if you look at the—even in the reaction equation, if you look at the structure of the beginning and the end you can assume the energy and you can assume how they will react and you can assume the forces and that kind of thing I guess … the farther we’ve gotten, the more important they’ve become to, like, know and understand and, like, understand all the components and then relating it to all the other ideas, like hybridization, and acids and bases and reactions and all that.”
While both Ruth and Shelly seem to recognize the importance of knowing how to draw Lewis structures, only Shelly expressed in detail how Lewis structures could be related to the core idea of structure–property without being prompted. Shelly talked about how she could use the Lewis structures to make predictions about the reactivity, energy, and even the “forces” of a molecule. Unfortunately, this type of description was in the minority, and most students did not express how Lewis structures could be used as a model to predict and explain chemical properties. This and the previous example highlight that, while it is important for students to practice skills, it is essential for students to also develop detailed and connected networks of knowledge that will allow them to use and apply their understanding in new scenarios.
Biology Example.
Similar to our findings in chemistry, little is known about what ideas students believe to be the big ideas for the biology course. For the chemistry big ideas, students seem to be mostly concerned with listing or talking about what some students later described as being more “technical” ideas or things learned. Whereas for many of the biology big ideas listed or stated, students seem to be relating them to their everyday lives and personal interests. A good example of this is observed when students talked about DNA as a big idea for the B1 course. When referring to DNA, students often talked about it in terms of its structure, but most importantly they talked about its “function” and “ability.” For example, many of the students talked about DNA as being the “basis for all life” (Tory), and how we “start off as a little DNA cell” (Zoe). DNA, along with DNA replication, transcription, and translation, can be related to the core idea of information flow, exchange, and storage. The core idea that “hereditary information is stored, used, and replicated” (Laverty et al., 2016) resembles many of the students’ descriptions when they talked about DNA and its replication, transcription, and translation.
Galen: “I mean obviously the main things, DNA carries information that make us who we are, what we look like… Well, the genes are what are passed down from your parents and all of your family and grandparents and stuff too. And the DNA is what actually is what’s made in your body. So actually makes how you look and yeah everything about you, hair color.”
Furthermore, these students’ descriptions revealed how they find the big ideas learned in the B1 course to be more relatable to their everyday lives, most likely as their biology and chemistry courses discuss phenomena at different scales (e.g., microscopic vs. macroscopic). When asked to compare their chemistry and biology experiences, some students discussed how they believed their chemistry knowledge helped them explain other things in biology that were related to their experiences.
As to why they listed DNA and DNA replication as their big ideas for their B1 course, there was a range of reasons. Two of the most common reasons, as found with chemistry, were re-occurring ideas and time spent on ideas (Figure 4). For example, students talked about how DNA and related ideas were big ideas because of how often they talked about them in the course:
Galen: “DNA for sure, because that’s always learned for the whole last half of the semester, pretty much just DNA. That’s definitely the biggest… I think everything else we learned, we kind of just stopped talking about it and hadn’t really referred back to it.”
When considering the reasons provided by students for their lists, it is important to think about what might have led them to such conclusions. For students to develop an expert-like level of meaningful understanding, they must connect, organize, and contextualize core ideas, which will allow them to use their knowledge in new situations. Bringing up the core idea of information flow, exchange, and storage over time by using examples such as DNA, replication, transcription, and translation, was perhaps the instructor’s way to help support students’ organization, contextualization, and construction of knowledge around the core idea.
The notion of big ideas being “identified by instructor” was unique for the B1 course (Figure 4). Given that students were explicitly told the big ideas for the course, it was not surprising to see a number of students who reported structure and function as a big idea of the course verbatim. However, it was interesting that many of these same students did not seem to be able to describe or even explain why this was a big idea. Consider Clarice’s description and reasons for listing the structure–function relationship as a big idea:
[Asked to say what she thinks the big ideas are for the B1 course]
“Oh [s/he] goes over this like every day. How structure determines function…It’s one of the ones [big ideas] s/he talks about like every day so that’s why it pops out of the top of my head.”
[Asked to say whether she agrees with her the instructor or not]
I’d say it’s up there, but sometimes I feel like he/she doesn’t explain himself/herself how s/he thinks it determines—like structure determines function. Like sometimes [s/he] gives a really good explanation and sometimes it’s like how do you get from here to here. So, I don’t know. Sometimes I see it, sometimes I don’t.”
[Student provides an example of what she thinks is structure–function]
“Maybe like cell structure and how different organelles and stuff work together, to perform a specific function. And if you think about like DNA, how it’s a double helix. So, like when you have to replicate it and what not, you have to tear it apart and kind of the processes. I don’t know [pause].”
Clarice is representative of many students who mentioned the instructor explicitly told them the take-home messages of the course when asked to provide a list of B1 big ideas. Furthermore, as previously reported, although structure–function seems to be the core idea “most internalized by students,” they were not always certain of what the instructor meant by “structure determines function” (Kohn et al., 2018a). Students like Clarice, who could recognize structure–function as a big idea, showed difficulties describing the idea, which often resulted in them providing examples of how they thought about the structure–function relationship. Similar to chemistry, the descriptions and reasons provided by the students on what they considered to be big ideas in biology are representative of the students interviewed.
Research Question 2: What Topics/Ideas Do Students Identify as Overlapping in Both Their Introductory Chemistry and Biology Courses?
In phase 3 of the interviews, students were asked to identify any perceived connections between the chemistry and biology courses (Figure 1) to explore students’ thoughts before they were asked specifically about energy and structure–function. As part of the follow-up portion of this study, surveyed students were asked to list “things learned” in one course that they found to be useful for a different course. That is, what things learned in GC1/GC2 they found to be useful to think about B1, or vice versa. From this point on, students’ responses for the connections between their courses, which included topics, concepts, ideas, and skills, are referred to as “overlapping ideas.”
The interviewed students provided an average of two to three overlapping ideas (see Supplemental Material S.12) between the courses; similarly, the surveyed students listed one to two overlapping ideas (see Supplemental Material S.13). All interviewed students were able to identify at least one overlapping idea, unlike in the survey, where a large number of students did not list any specific connections between the courses. In particular, 44% (n = 51) of these students found it difficult to identify any specific ideas in biology that would help them with their understanding of chemistry, thus they were coded as “No specific idea provided.”
Student Survey 222: “Chemistry helped me with biology, but biology didn’t help me with chemistry.”
Student Survey 272: “[B1] was a[n] elaboration of [GC1/GC2], so I did not think that topics learned in biology helped me in chemistry.”
Student responses to the survey showed that they considered chemistry ideas to support their understanding of biological phenomena and systems, but they found it much more difficult the other way around. In fact, only 13% (n = 15) of the surveyed students were coded as “No specific ideas provided” when asked if they perceived any ideas from chemistry that supported their understanding of biology, indicating that the majority of the students were able to perceive at least one connection between what they learned from chemistry to apply to biology. From the surveyed students, 11 of them said things like “None,” “I can’t think of any,” or “It was completely separate,” and four of the students were coded as “No specific ideas provided”; however, they found most of the chemistry ideas to be helpful for their understanding of biology.
Student Survey 302: “Honestly majority of the course was helpful.”
Student Survey 522: “Basically everything we learned helped me to understand biology because they are both very connected.”
These students who thought the “majority” or “everything” from the chemistry course had helped with their biology understanding are similar to the interviewed students who perceived chemistry to be the basis of biology (21%, n = 6, Table 3).
| Top 5 overlapping ideas | |
|---|---|
| Ideas listed during the interview | Overall: no. of students (%) |
| Interactions | 7 (25) |
| Types of bonds | 7 (25) |
| Chemistry is the basis of biology | 6 (21) |
| Polarity | 6 (21) |
| Structures | 6 (21) |
| Ideas listed during the survey | GC1/GC2 to B1 no. of students (%) |
| Interactions | 49 (45) |
| Reactions | 37 (34) |
| Types of bonds | 24 (22) |
| Gibbs free energy | 15 (14) |
| Energy | 12 (11) |
| Ideas listed during the survey | B1 to GC1/GC2 no. of students (%) |
| Reactions | 15 (14) |
| Enzymes | 11 (10) |
| ATP | 11 (10) |
| pH | 8 (7) |
| Gibbs free energy | 7 (6) |
Natalie: “I’d say that—I mean obviously there’s differences between chemistry and biology in what you’re studying… But I’d say that biology is almost an extension of chemistry or what we’re learning in chemistry. So you have to take all the properties that you’ve learned and actually apply that to these different living systems and so without really understanding the chemical—like what you learned in chemistry, it would be difficult to understand biology, like and actually understand it instead of just memorize it I would say. I feel like chemistry is a little bit more of the raw basis of what you’re learning so you don’t necessarily need to know biology to know chemistry, but I feel like you should know chemistry in order to understand biology, almost. And so I would say that they’re interrelated in that way.”
Students like Natalie were able to recognize chemistry as being of value and playing a crucial role in explaining biological systems. This is an important finding, given that a core idea in biology is the chemical and physical basis of life—that is, chemical and physical interactions and reactions result in life processes.
Furthermore, both interviewed and surveyed students identified the ideas of interactions, reactions, and type of bonds as overlapping ideas between the courses (Table 3). Students’ recognition of these ideas as important overlapping ideas is a positive finding, which suggests that they have recognized how the ideas learned in individual courses could support their understanding of phenomena in other disciplines. This is particularly true for the idea of interactions, which was recognized by at least 25% (n = 7) of the interviewed students and 45% (n = 49 the GC1/GC2 to B1 list) of the surveyed students as an important overlapping idea. It is also important to note that students’ explanations in the interviews of how interactions overlapped between their courses ranged from listing the type of interactions to comparing how interactions are talked about in each course.
Galen: “Just intermolecular forces and bonding. That’s about it… Yeah. Like the way we learned about hydrogen bond is more technical in chemistry, but the hydrogen bond in biology is not as technical but they still don’t contradict each other.”
Karl: “Everything. Absolutely everything, I guess. Like everything in biology is a molecule. All biology is an interaction of like complex molecules. Okay, take like buffers, I guess, as an example—carbonic acid. When CO2 is released it messes around with H2O and it becomes carbonic acid. And this is an acid. If our blood didn’t have a buffer system, we would probably die because this acid’s building up. Things like that. You definitely have got to know a lot about chemistry to do biology or else you’re going to be in trouble.”
As can be observed from these quotes, students like Galen thought learning about IMFs in chemistry was more “technical,” meaning he learned about how and why interactions occur, while he could see in biology examples of the IMFs learned in chemistry. Students like Karl, however, provided more detailed explanations on how they saw the idea of interactions as playing a central role in biological processes. These results are similar to those previously reported (Kohn et al., 2018b) on how students perceived the core ideas of energy and structure–function (phase 4 of the interviews; Figure 1) for different disciplines (chemistry vs. biology). Findings from that work showed that, while students could make connections between disciplines, their views and understandings of these ideas varied depending on the course invoked. Overall, results from the study presented herein show that, even though students were not able to provide many overlapping ideas between the courses, our findings suggest that the ideas students recognized as overlapping are essential and play a central role in their understanding of phenomena in both courses. See Supplemental Material S.12 and S.13 for the full list of overlapping ideas provided by students.
Summary
The interviews conducted in this study were inspired by the vision laid out in the Framework for K–12 Science Education for what students should know and be able to do with their scientific knowledge (NRC, 2012a). As part of the study, students were asked to provide lists of things (which could encompass facts, topics, concepts, skills, core ideas) learned throughout both their GC1/GC2 and B1 courses and also what they perceived were the big ideas for said courses. Given the nature of the courses at this institution, where emphasis was placed on a set of core ideas as part of both curricula, the expectation was that students would bring these ideas up. In fact, the results from both the interviews and surveys for research question 1 showed that students presented many ideas for each course, some directly related to the courses’ core ideas, while others have the potential to be related to a core idea, although the ways that students discussed their choices were somewhat different, depending on the discipline. For example, students in chemistry talked about big ideas that can be related to the core ideas of electrostatic and bonding interactions through their discussions of different type of interactions and bonding, and they also referred to atomic/molecular structure and properties as they used structures to predict reactivity and other properties. In the context of biology, students identified and discussed core ideas including information flow, exchange, and storage, evolution and structure and function. While the interviews provided richer and more in-depth descriptions on how students thought about the big ideas listed, the findings from the survey revealed that the larger group of students also considered similar ideas to be the take-home messages of the courses, particularly the ideas of interactions and reactions in chemistry and concepts related to cell structure in biology.
When students were interviewed about their reasons for specifying big ideas, commonalities identified among the responses included the amount of time spent on an idea or how often the idea came back within the course (Figure 4). However, some of the students’ rationalization for selecting big ideas appears to differ by discipline. For example, explanatory and predictive power was far more prevalent for chemistry, while identified by instructor and basis for other ideas were more common for biology; however, re-occurring ideas was stated by students as an important reason for both disciplines. The disciplinary difference was also apparent in the ways that students linked particular ideas with their reasoning for selecting them as big ideas. As exemplified by quotes provided by Galen and Laura for the general chemistry sequence, the core idea of interactions was frequently emphasized throughout, while Karl discussed how interactions can have explanatory and predictive power. However, in the B1 course, for the core idea of information flow, exchange, and storage, students focused more on the associated ideas or topics that could fall under this core idea (i.e., DNA, DNA replication, transcription, translation) and examples provided in class. For the core idea of structure and function, students certainly were able to identify the “phrase” as a core idea but appeared to have difficulties with its contextualization. This difference between the two courses may well be a reflection of the different stages of transformation of the two courses as discussed in Implications for Teaching.
The findings from research question 2 show that, when students were asked to make explicit connections between their chemistry and biology courses, they were able to identify productive common ideas. Generally, students identified an average of two or three overlapping ideas during the interviews and surveys, which means that these could be used by instructors to support and facilitate connection between disciplines. In general, students were more likely to indicate that chemistry ideas were useful in biology, rather than vice versa. This is not surprising, indeed the biology course has a prerequisite of at least one chemistry course, while the chemistry course has no biology prerequisite.
IMPLICATIONS FOR TEACHING
As previously noted, the two courses in this study were undergoing a transformation aligned with the vision of the Framework; however, the transformation of the two courses was at different stages, and the two instructors took a somewhat different tack as they implemented each transformation. The general chemistry course (CLUE) was a completely redesigned course intended to weave the four core ideas of chemistry into the course framework. That is, there were no chapter or topic headings labeled “Interactions,” or “Structure–Property Relationships,” or “Energy.” Instead, these core ideas were embedded in the fabric of the course. The instructor did not explicitly discuss core ideas, as the course was organized in such a way that each topic was linked to them. In contrast, the biology course used a commercial textbook, with traditional topic/chapter headings, and the instructor explicitly discussed what the core ideas of the course were and how they were linked to the day’s topic. This difference may account for the fact that very few biology students discussed big ideas as being explanatory and predictive, while no chemistry students gave the fact that the teacher named the big ideas as a reason for stating them.
The role of core ideas as envisaged in the Framework is that they undergird instruction and can be used to explain and predict a wide variety of phenomena. This is in contrast to topics, which often correspond to chapter headings or even a particular phenomenon. Core ideas can connect students’ knowledge, and make it accessible, if they are developed over time throughout the curriculum. On the other hand, if a particular core idea is treated as a topic, it will not serve this purpose, and it will be more difficult for students to make connections across ideas and phenomena. If faculty want students to understand core ideas in this more general way, it will be important to do more than simply introduce them as if they were another topic or chapter heading.
The findings presented in this paper highlight the importance of being explicit about not only introducing core ideas, but also emphasizing their connections to phenomena during instructional activities. Carefully introducing such ideas and providing students with assessments that support the development of connected and integrated ideas will enable students in developing more expert-like understanding of the discipline. Instructors should also provide explicit opportunities to support students in their understanding of core ideas. These ideas need to be introduced in a consistent manner and returned to as often as possible to help students strengthen connections between core ideas and topics.
We have also shown that, while students believe that chemistry ideas can be used in biology, the reverse is not necessarily true. If we want to support students’ connected understanding, this should be a two-way street; we as instructors must work to emphasize these connections, particularly in chemistry courses where the majority of students are biology majors. Students must be provided with explicit opportunities to make interdisciplinary connections that support their use and transfer of knowledge across disciplines. This may take slightly different forms in the two courses. While it seems clear that the core ideas in chemistry can be used to explain and predict some types of biological phenomena, such as hydrogen bonding in base pairs or protein folding to enzyme substrate interactions, the reverse is not true for the core ideas in biology. The biology core idea “the chemical and physical basis of life” has a clear connection to the chemistry core ideas. However, the “cellular basis of life” or “information flow and storage” do not have obvious connections to chemistry ideas (although, of course, the mechanisms by which these core ideas operate are still explained and predicted at a molecular level by chemistry core ideas). Therefore, perhaps we should not be surprised that students indicated that biology was not necessary to understand chemistry.
While chemistry can be taught without reference to biology, this does not mean that it should be taught this way. Biological examples would serve to consolidate chemistry ideas, and providing such context might make chemistry more interesting for many students. While biological systems are more complex than the ones typically discussed in a general chemistry course, they could certainly be used as the “end goal” for instruction. For example, when learning about intermolecular attractions, DNA base pairing is an obvious extension, or when learning to draw structures, simple biological molecules can be the target.
Finally, our findings have implications for the order in which the chemistry and biology courses are taught. Our study highlights how students were able to identify a larger number of chemistry ideas that they found useful to support their understanding of biological phenomena, while it was much more difficult for them to identify biology ideas that would support their understanding in chemistry. Therefore, this study provides evidence that the order in which chemistry and biology courses are taken does have some impact on how students use knowledge from one course to apply toward another course. Here, the student responses indicate that chemistry courses being taught before biology may lend a better knowledge framework of understanding for their chemistry knowledge to be used and applied in their biology courses.
LIMITATIONS
The results presented here were gathered at a single institution where both the chemistry and biology courses had either been transformed or were in the process of being transformed. Instructors in both courses valued and placed large emphasis on core ideas. Therefore, findings from a different time point of the transformation within the same institution, different institutions, or courses using a different curriculum might result in different findings from the ones presented here. In addition, students were not asked to provide a description and explanation for each of the topics/ideas listed during the interviews; therefore, future research would be warranted to further explore these ideas and gain a better understanding on how students are using the ideas listed. Furthermore, additional research is needed to understand why students listed individual ideas as being important for one discipline only but struggled to identify potential connections when asked to purposely think about how the two disciplines overlapped.
ACKNOWLEDGMENTS
We thank all the students who participated in the interviews and completed the survey and their instructors for granting us access to their courses. We also thank the chemistry education research team at FIU for their input and support throughout the project. In addition, we would like to thank Drs. Timothy N. Abell and Maia Popova for their input and assistance on the development of some of the figures. This work was supported in part by the Association of American Universities’ (AAU) Undergraduate STEM Education Initiative, funded by the Helmsley Charitable Trust, and the National Science Foundation grants DUE 0816692 (1359818), 1708664, 1708589, 1725609, and 1725520. Any opinions, findings, conclusions, or recommendations expressed here are those of the authors and do not necessarily reflect the views of the National Science Foundation or other funding sources.